October 07, 2025
6 min read
Key takeaways:
- Fewer adults diagnosed with PAH less than 1 year ago receiving add-on Winrevair vs. placebo had at least one qualifying clinical worsening measure.
- The drug’s safety profile was consistent with what is known.
Winrevair added to background therapy decreased the risk for clinical worsening in adults diagnosed with pulmonary arterial hypertension less than a year ago, according to data published in The New England Journal of Medicine.
These findings on Winrevair (sotatercept, Merck) were also simultaneously presented at the European Respiratory Society International Congress.

Data were derived from McLaughlin VV, et al. N Engl J Med. 2025;doi:10.1056/NEJMoa2508170.
“The compelling results of the HYPERION study demonstrated that initiation of Winrevair on top of background therapy within the first year of diagnosis significantly reduces the risk of clinical worsening events compared to placebo,” Vallerie V. McLaughlin, MD, Kim A. Eagle, MD, Endowed Professor of Cardiovascular Medicine and director of the pulmonary hypertension program at University of Michigan in Ann Arbor, said in a press release.
In the global, double-blind, parallel-group, placebo-controlled, randomized phase 3 HYPERION trial, McLaughlin and colleagues evaluated 320 adults diagnosed with PAH functional class II or III less than 1 year ago (median, 7 months) to determine the impact of Winrevair added to double or triple background therapy vs. placebo on clinical worsening defined as “a composite of death from any cause, unplanned hospitalization lasting at least 24 hours for worsening of PAH, atrial septostomy, lung transplantation or deterioration in performance in exercise testing due to PAH.”
Notably, patients needed to have an intermediate or high risk for death, according to the study.
As Healio previously reported, this trial was stopped early due to robust positive evidence found in prior trials of the activin-signaling inhibitor.
Among those receiving Winrevair (n = 160; mean age, 57.3 years; 75% women; 87.5% white; 60% from Europe), researchers outlined that the drug was delivered subcutaneously every 21 days, at a starting dose of 0.3 mg per kg of body weight and escalated to the target dose of 0.7 mg per kg. The remaining 160 patients (mean age, 55 years; 70% women; 85% white; 61.2% from Europe) received placebo.
Clinical worsening findings
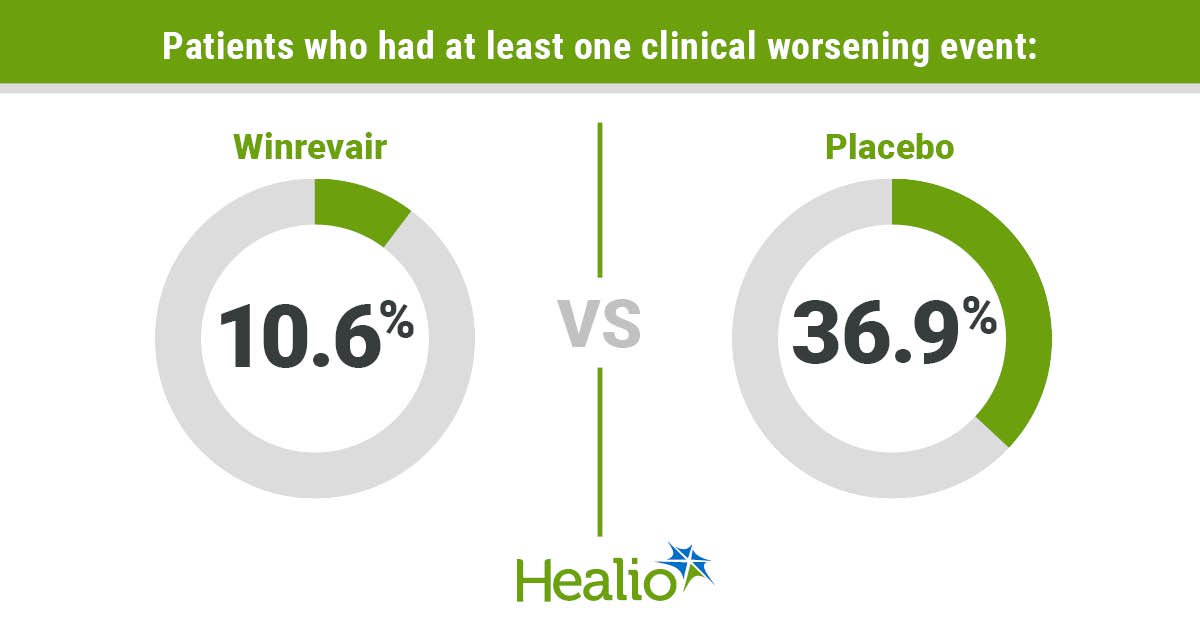
Over the median follow-up period of 13.2 months, the study reported that more patients receiving placebo vs. Winrevair had at least one of the clinical worsening measures (n = 59 [36.9%] vs. n = 17 [10.6%]), and the Winrevair group had a significantly reduced risk for this outcome (HR = 0.24; 95% CI, 0.14-0.41).
Researchers continued to find this result when patients were separated based on PAH subtype (idiopathic PAH, connective tissue disease), background PAH therapy (double or triple) and risk level (intermediate risk using the REVEAL Lite 2 risk score or intermediate-low risk using the COMPERA 2.0 risk score).
When analyzing Kaplan-Meier curves, separation between the two groups was “early and sustained,” demonstrating clinical benefit with Winrevair after three doses, according to the study.
In total, researchers observed 84 events indicative of clinical worsening, four of which were not first events. When broken down into its individual factors, the most reported event was deterioration in performance in exercise testing due to PAH in both groups (placebo, n = 46 [28.8%]; Winrevair, n = 8 [5%]).
In terms of unplanned hospitalization for worsening of PAH, 14 patients receiving placebo (8.8%) and three patients receiving Winrevair (1.9%) had this outcome. A similar proportion of patients from each group died from any cause (placebo, n = 6 [3.8%]; Winrevair, n = 7 [4.4%]), according to the study.
For the remaining two factors of atrial septostomy and lung transplantation, researchers observed no cases of either in both groups.
Among those who reached week 24 at the time of the early trial termination (n = 287), the study noted that significantly more patients receiving Winrevair vs. placebo had multicomponent improvement (29.4% vs. 14.6%; treatment estimate, 14.45; 95% CI, 5.12-23.92) and maintained or achieved a low REVEAL Lite 2 risk score (60.1% vs. 47.9%; treatment estimate, 12.34; 95% CI, 0.83-23.54).
In contrast, there was no significant difference in the percentage of patients with a low simplified French risk score at week 24 when comparing the two groups.
“Although the third secondary endpoint (Simplified French Risk Score) was not significant and subsequent endpoints were not tested, improvements in WHO functional class, 6MWD and NT-proBNP were consistent with prior study results,” according to the presentation.
Safety
Over the median follow-up period of 14.6 months in the Winrevair group, 89.4% of patients reported an adverse event, and this was similar to the 90% of patients in the placebo group who reported an adverse event during a median follow-up period of 11.5 months, according to the study.
Researchers highlighted four notable adverse events that occurred in a greater proportion of patients receiving Winrevair vs. placebo: epistaxis (31.9% vs. 6.9%), telangiectasia (26.2% vs. 11.2%), bleeding events (41.2% vs. 16.2%) and an increased hemoglobin level (11.2% vs. 1.2%).
Further, the study noted that more patients receiving Winrevair vs. placebo had an adverse event leading to treatment discontinuation (3.1% vs. 0%) and an adverse event considered to be related to the treatment (57.5% vs. 30%); however, fewer patients receiving Winrevair reported serious adverse events (24.4% vs. 28.1%).
Both groups had a similar, small proportion of patients who died due to an adverse event (Winrevair, 2.5%; placebo, 3.1%), according to researchers.
The release stated that Winrevair’s safety profile in this trial “was generally consistent with that observed in previous studies.”
“These positive results from HYPERION expand on the body of clinical evidence for Winrevair, now including PAH patients within their first year of diagnosis, including those earlier in their treatment journey,” Joerg Koglin, MD, PhD, senior vice president, head of general and specialty medicine, global clinical development at Merck Research Laboratories, said in the release. “The totality of Winrevair data to date continues to reinforce our confidence in its practice-changing potential.”