December 30, 2025
2 min read
Key takeaways:
- Adults receiving semaglutide in the SELECT trial had a lower risk for any hospitalization than those receiving placebo.
- The semaglutide group spent fewer days admitted to the hospital than the placebo group.
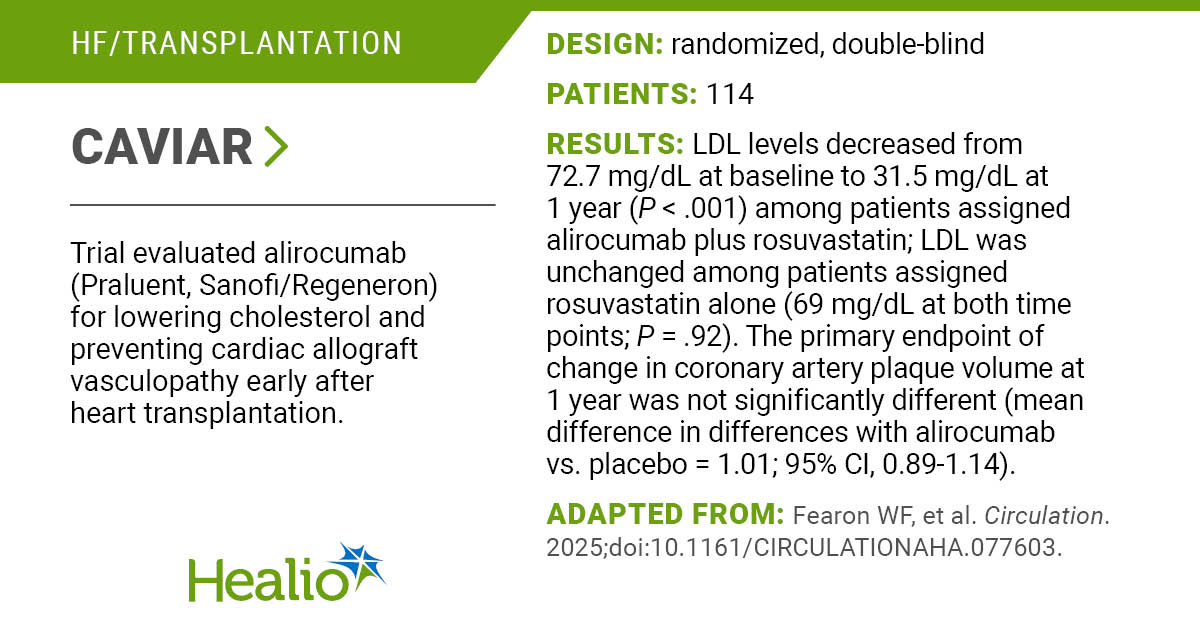
Once-weekly subcutaneous semaglutide 2.4 mg reduced the risk for any hospitalization among adults with obesity and established cardiovascular disease, according to an analysis of the SELECT trial published in JAMA Cardiology.

Stephen J. Nicholls
“The benefits of semaglutide (Wegovy, Novo Nordisk) in the SELECT trial extend far beyond the reduction in CV events that were previously reported,” Stephen J. Nicholls, MBBS, PhD, professor of cardiology and director of Victorian Heart Institute at Monash University in Melbourne, Australia, told Healio. “We observed a reduction in hospital admissions for a range of indications. The degree to which that [reduction] reflects the benefits of weight loss or other effects of semaglutide remains to be determined.”

Once-weekly semaglutide 2.4 mg reduced the risk for any hospitalization among adults with obesity and CVD in the SELECT trial. Image: Adobe Stock
As Healio previously reported, the SELECT trial enrolled 17,604 adults with obesity and established CVD (median age, 61 years; 27.7% women). Participants were randomly assigned, 1:1, to once-weekly semaglutide 2.4 mg or placebo. In the exploratory analysis, researchers collected total hospital admissions and days spent in hospital for all participants.
During a median follow-up of 41.8 months, 35% of the study group was admitted to the hospital, 31.8% were hospitalized due to a serious adverse event and 6.8% were admitted for an elective hospitalization.
Adults receiving semaglutide had a lower risk for any hospitalization (HR = 0.89; 95% CI, 0.84-0.93; P < .001), a hospitalization due to a serious adverse event (HR = 0.88; 95% CI, 0.84-0.93; P < .001) and an elective hospitalization (HR = 0.86; 95% CI, 0.77-0.97; P = .01) than those receiving placebo. The number of days spent in the hospital were similar between the two groups.
The semaglutide group was less likely to be admitted to the hospital for a cardiac event (HR = 0.83; 95% CI, 0.76-0.91; P < .001), infection (HR = 0.85; 95% CI, 0.76-0.95; P = .004) or a respiratory event (HR = 0.66; 95% CI, 0.54-0.8; P < .001) than the placebo group. Adults receiving semaglutide were less likely to undergo a surgical or medical procedure vs. the placebo group (HR = 0.81; 95% CI, 0.71-0.92; P = .001).
Trial participants had 11,287 total hospital admissions during follow-up. The semaglutide group had 18.3 total admissions per 100 patient-years vs. 20.4 total admissions per 100 patient-years with placebo (P < .001). Adults receiving semaglutide had 15.2 admissions for serious adverse events per 100 patient-years compared with 17.1 admissions per 100 patient-years for the placebo group (P < .001). The semaglutide group spent fewer days in the hospital for any event (157.2 per 100 patient-years vs. 176.2 per 100 patient-years; P = .01) and for serious adverse events (137.6 per 100 patient-years vs. 153.9 per 100 patient-years; P = .02) than the placebo group.
“Semaglutide has a range of metabolic benefits including weight loss and favorable effects on glycemic control, blood pressure, lipids and inflammatory markers,” Nicholls said. “These findings further suggest that semaglutide has the potential to benefit a range of health outcomes in high-risk patients with overweight and obesity.”
For more information:
Stephen J. Nicholls, MBBS, PhD, can be reached at stephen.nicholls@monash.edu or on X @ProfSNicholls.