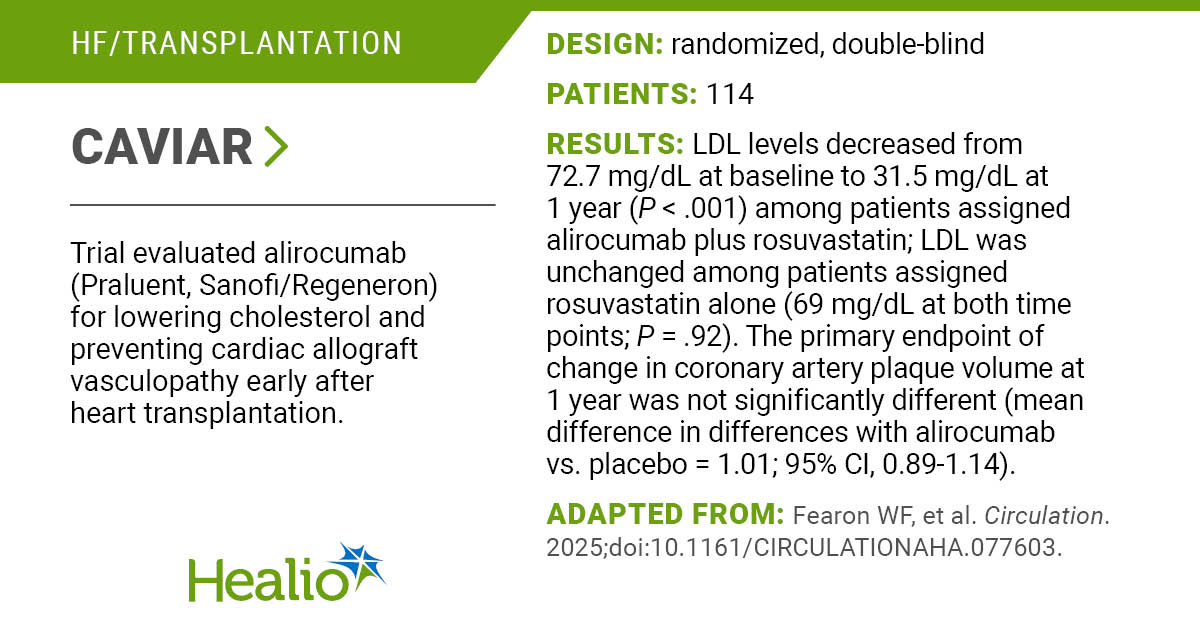
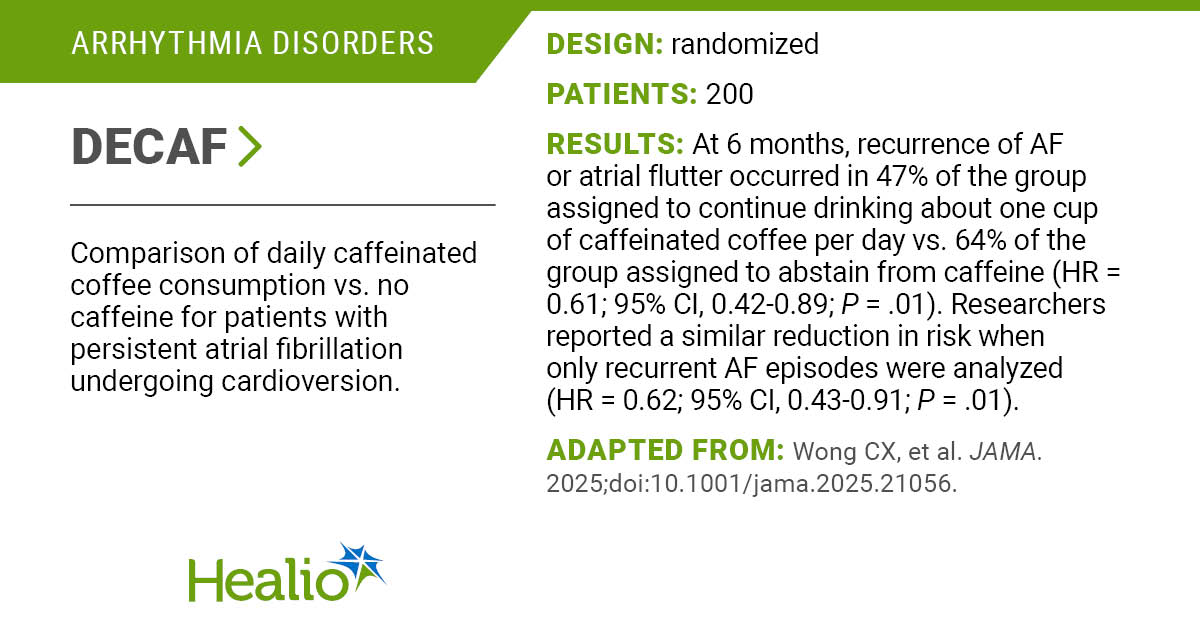
WASHINGTON — Vinay Prasad is returning to the Food and Drug Administration to resume his role overseeing vaccine, gene therapy, and blood product regulation.
“At the FDA’s request, Dr. Vinay Prasad is resuming leadership of the Center for Biologics Evaluation and Research,” Health and Human Services spokesperson Andrew Nixon told STAT on Saturday.


This article is exclusive to STAT+ subscribers
Unlock this article — plus in-depth analysis, newsletters, premium events, and news alerts.
Already have an account? Log in