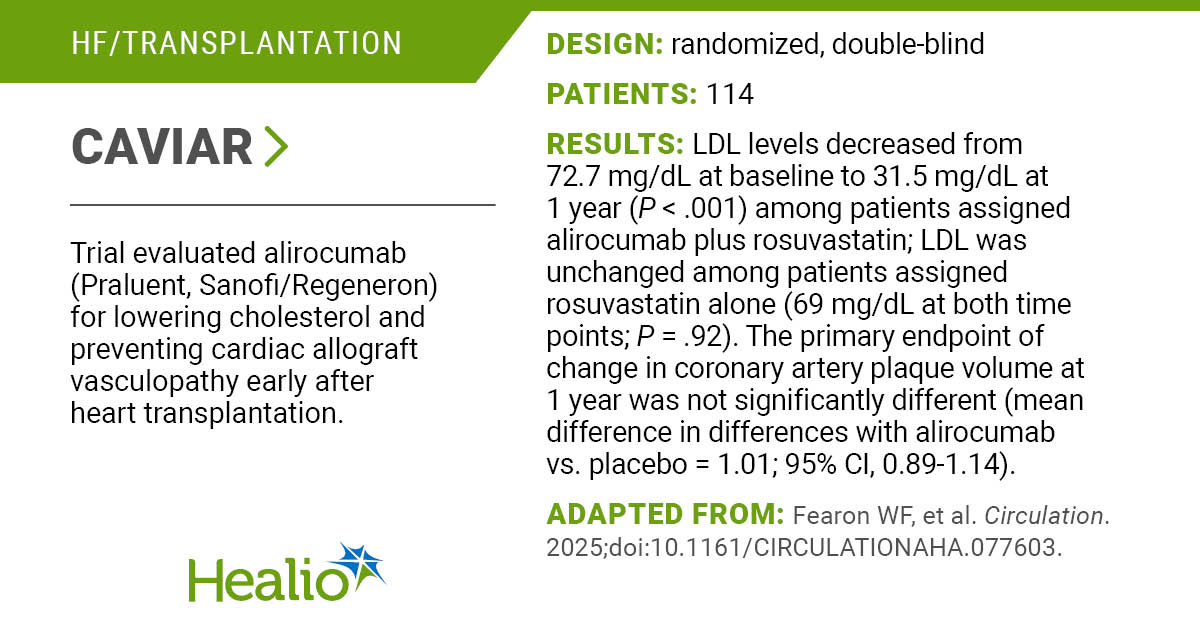
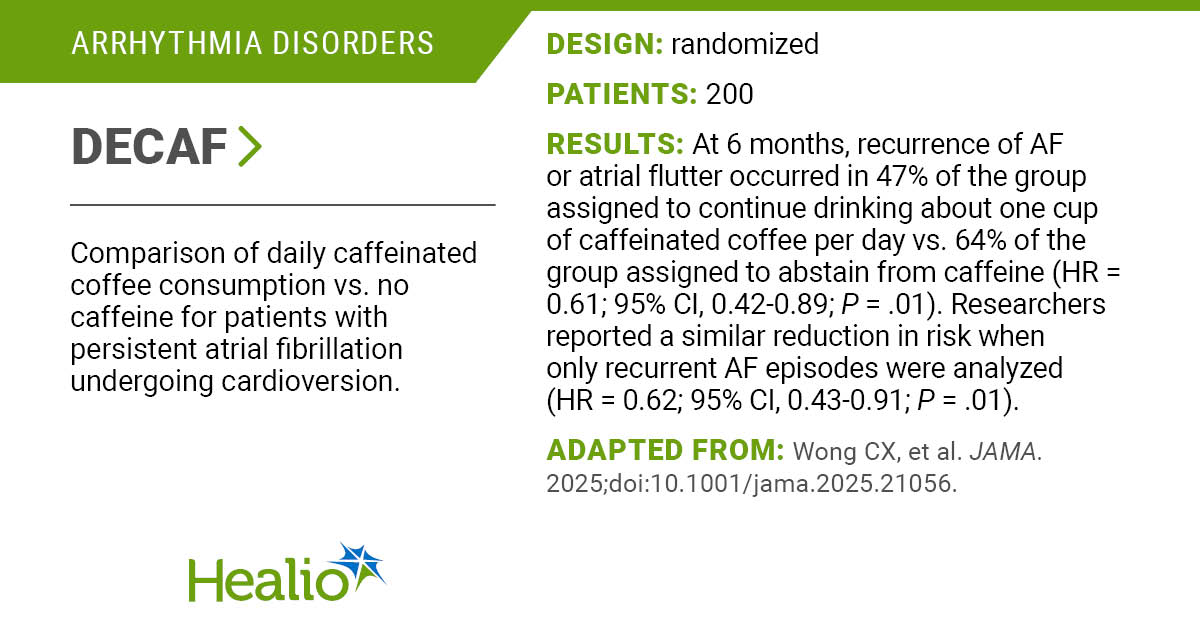
The Trump administration is proposing a voluntary program in which pharmaceutical companies can offer rebates for a small number of therapies to hospitals that participate in a federal drug discount program, an effort to sort out a controversy that has roiled the health care industry.
At issue is the 340B discount program, which was created to help hospitals and clinics care for low-income and rural patients. Drug companies that want to take part in Medicare or Medicaid must offer their medicines at a discount — typically, 25% to 50%, but sometimes higher — to participating hospitals and clinics. Right now, drug companies generally provide the discounts at the time of purchase.
Since its inception more than three decades ago, the program has ballooned — there are now about 55,000 participating entities — and has regularly factored into the clash in the U.S. over drug prices. Prescription medicines purchased under the program amounted to $66.3 billion in 2023, a 23.4% increase from the previous year, according to federal government data.


This article is exclusive to STAT+ subscribers
Unlock this article — plus in-depth analysis, newsletters, premium events, and news alerts.
Already have an account? Log in