This post by Peter Selley centres on a Moderna RSV vaccine trial, the Rhyme trial, in young babies that was stopped last year when 7 out of 40 babies between 5 and 8 months of age developed severe lung disease, compared to 1 in 20 controls.
Moderna had good reason to think its vaccine could cause this, as you will see, but the company appears to have opted to overlook the pointers to a problem it had found before their trial began.
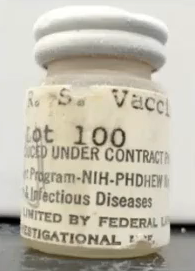
Read on to find out how the disturbing image above links to vaccine trials.
The RSV Challenge
RSV has been a graveyard for vaccines since the 1960s when children died because of vaccine associated enhanced respiratory disease (VAERD). Before that, successful vaccines for smallpox, yellow fever, tetanus and diphtheria suggested making a vaccine for the just discovered RSV virus should be easy.
A Formalin inactivated vaccine, Lot 100, made RSV antibodies in the infants but many of the vaccinated children developed severe chest infections. Two boys died – from the virus they were supposed to be protected from.
We now know VAERD affects RSV naïve infants. If you get the vaccine before acquiring natural immunity, you risk getting it. If you get the vaccine later, and it’s a safe bet that after the age of 1 you will have had RSV, there is no problem.
There are four ways a baby can get immunity to RSV:
- All babies are born with low levels of RSV antibodies, resulting from repeated RSV infections during the mother’s life, transferred in utero or by breastfeeding.
- Babies can be immunised after birth with monoclonal antibodies e.g. Beyfortus (nirsevimab). This gives massive antibody levels in the baby’s bloodstream.
- Babies can be exposed to a maternal RSV vaccine in utero, which can produce massive maternal and placental antibody levels.
- Babies can catch RSV after birth and develop their own natural antibodies to the virus.
The best way to develop immunity is to acquire it naturally. “Vaccine” antibodies are not the same as the naturally acquired ones.
Unlike Measles or Chickenpox, catching RSV doesn’t give long-term immunity. You are going to get cold-like symptoms lasting a few days every few years. Which means children can be lined up to get vaccines every year or two, which pharma companies are happy to support.
Some infants who catch RSV can develop bronchiolitis which can cause wheeziness. It is generally mild. The public health problem with RSV is that children with bronchiolitis frequently get admitted to hospitals – often just for one night. A very small number become seriously ill, and there are occasional deaths – mainly in children with an underlying disease. 97% of deaths are in low to middle income countries in Africa. In temperate regions like Europe, North and South America and Australia there are invariably outbreaks each winter.
The VAERD Challenge
VAERD led to a virtual embargo on creating traditional RSV vaccines for young non-immune babies. VAERD is a hazard of many respiratory vaccines – not just RSV. Dengue is the most famous. VAERD is a hazard Anthony Fauci says we don’t understand. There were, and still are, concerns that Covid vaccines could cause it.
Efforts to overcome the problem have taken several different approaches including;
- Targeting a prefusion F-Protein (PreF) on the RSV virus.
- Producing a DNA vaccine – as with Astra-Zeneca’s DNA Covid vaccine.
- Producing an mRNA vaccine – as with Moderna’s SpikeVax
- Vaccinating the pregnant mother
- Giving a monoclonal antibody to the newborn infant.
Novavax, allied to Fernando Polack and Bill Gates, ran a maternal PreF protein vaccine trial. They decided it was not efficacious enough to warrant pushing all the way through to marketing – although there was little difference between the results for this and a very similar PreF vaccine which Pfizer did push through to marketing – and semi-mandating.
GSK gave a DNA vaccine to infants over the age of 1, who had already had RSV – none developed VAERD. In mostly RSV naïve infants between 6 and 12 months, however, it produced lots of respiratory infections and other problems but GSK claim no VAERD. But they also say that Covid lockdowns meant there was no RSV around, which makes it difficult to get VAERD.
GSK abandoned this DNA vaccine for a maternal PreF vaccine, which had minimal benefits like Novovax and Pfizer’s. This too was abandoned as it was associated with an excess of premature births and infant deaths.
Pfizer’s Abrysvo is a PreF vaccine, almost identical to GSK’s, given to pregnant mothers rather than infants. Its efficacy is close to indistinguishable to Novovax and GSK. It is linked to maternal hypertension, and preterm births – problems that compromise the health of women and their children for the rest of their lives.
AZ Sanofi’s Beyfortus is a monoclonal antibody injected into newborns. It gets called a vaccine, but it isn’t. Its safety profile is still unclear – it appears linked to infant deaths including respiratory deaths.
Abrysvo and Beyfortus have been licensed. These are end-runs around VAERD rather than solutions to the problem. And, on the basis that if we don’t talk about it, it won’t exist, the term VAERD is disappearing, replaced by ERD. The vaccine link is now censored.
The licensing of Arbysvo and Beyfortus is a problem for attempts to solve VAERD and make a better RSV vaccine. Loads of RSV vaccines are in the pipeline.
But how can any of these break into the market if mothers or infants are mandated to get something else? Something else whose real world safety has not been established?
Cotton Rats
VAERD can be mimicked in cotton rats, immunised with a vaccine and then challenged with RSV. This makes it possible to test potential vaccines for VAERD safety.
In 2017, Medimmune (AstraZeneca) developed a traditional vaccine based on the RSV F Protein. Aware of VAERD, they ran tests in cotton rats, some of whom developed signs of lung disease. The vaccine was ditched. The investigators warned:
The safety signals for ERD observed in the antigen de-escalation studies raise concerns about the safety of an RSV post-F/pre-F plus adjuvant subunit vaccine in the RSV-naive pediatric population. It is critical that vaccines developed for RSV-naive populations do not represent a risk for ERD at the proposed pediatric dose, and vaccine developers need to also consider the risk for ERD with suboptimal dosing, which might occur in clinical practice and with waning of the immune response. As several non-live vaccine candidates are in preclinical and clinical development, the findings presented here are of utmost importance to guide preclinical evaluation of vaccine candidates and to ensure the safety of RSV-naive infants and children.
Flushed with the success of Spikevax for Covid-19, Moderna tweaked their mRNA platform to induce immunity towards the PreF protein in two bronchiolitis viruses.
mRNA-1345, mResvia, aimed at the RSV Pre-F protein. In addition to being tested in children, mRESVIA was approved for adults over 60 and scheduled for the 2024-2025 RSV season.
mRNA-1365 targeted both RSV and hMPV. Human Metapneumovirus has featured in feverish articles in the media that get feverish about viruses and vaccines – a terrible new disease is apparently sweeping China. In fact, it is a mild ‘cold’-type virus.
Moderna’s Rats
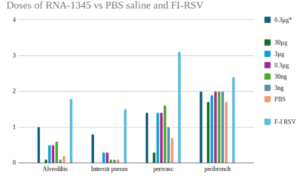
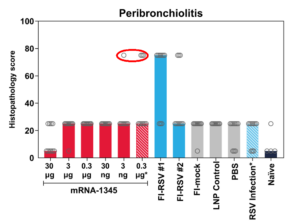
Moderna tested their mRNA-1345 in cotton rats. They injected different vaccine doses into the animals, blew RSV virus into their lungs and examined lung tissue five days later. The results were published as an unreviewed preprint – linked above – and this Supplement.
The extent of the lung damage was presented in terms of median effects. That is they were averaged which in this case is plain wrong – it is a way to hide “outlying” results. Even a few instances of severe lung damage in vaccinated rats are vitally important, especially if they are more frequent than placebo.
The results are charted in figure 5 in the link above. According to the preprint lung tissue damage was scored blindly on a 0–4 severity scale and subsequently converted to a 0-100% histopathology scale (0 = 0%, 1 = 5%, 2 = 25%, 3 = 75%, 4 = 100%). Initially, thinking this was a typo, I contacted Dr Shaw, the author, asking if a score of 2 out of 4 equated to 25%. I also requested numeric data used for the charts. No reply.
I redid the Shaw tables abstracting the numbers from her charts, using the mean of the original scores rather than a median of the “percentages”.
The high pale blue bars to the right of each section show you the Lot 100 data. The dark green bars to the left of each section show the results for the medium sized dose of mResvia given once.
The mRNA vaccine has different kinetics to the original Lot 100 vaccine. The graphs might show more damage if the rats been sacrificed a few days later.
A key question is were any animals badly affected? Did they have an equivalent of VAERD? Does this study show that mRNA-1345 has none of the dangerous effects of the Formalin inactivated vaccine?
In almost every instance there are more signs of inflammation in the alveoli, peribronchioles and other lung tissues in the vaccinated than in the placebo groups.
But in addition, this figure shows that there were some severely affected animals. There should have been more detailed experiments.
The Panama Papers
Moderna’s RSV trials were supposed to take place in Australia, Canada, Panama, Poland, South Africa, Spain, the U.K., and U.S.. Only babies in the US and Panama plus One from the UK had been recruited before the trial was stopped.
An initial trial began in children aged 8-23 months – 25 recruited from USA and 65 from Panama. US children were compensated up to $2400.
Dosing started between February and June 2023, and was completed by October 2023
The vaccine was no better at preventing symptomatic RSV disease compared with placebo with 40% of the vaccinated babies developing RSV by October. One vaccinated child was later admitted to hospital with RSV pneumonia.
In March 2024, a Data Safety Monitoring Board gave the go-ahead for a second trial in 60 younger babies (5-7 months) who were recruited in Panama only. This started in May 2024.
By July two vaccinated babies had been hospitalised with lung disease. This stopped the trial. This table gives the incidence of serious lung disease in babies. Did the serious cases have VAERD?
| mRNA-1345 | mRNA-1365 | Placebo | |
| N | 20 | 20 | 20 |
| hMPV | 0 | 2 | 0 |
| RSV | 2 | 3 | 1 |
FDA convened a Vaccines and Related Biological Products Advisory Committee to discuss the Moderna trial on 12 December 2024. The agenda was published 48 hours before the meeting, effectively stifling public comment.
By coincidence, the day before the FDA meeting, two unreviewed preprints about the Moderna vaccine were published online. The first described the rat studies above.
A second Matthew Snape et al preprint described the clinical features of the babies who reacted badly to the trial vaccine. Snape had given up a job at Oxford University as Professor in Paediatrics and Vaccinology in 2022. He joined Moderna UK as Vice President, Clinical Development, for Maternal and Paediatric Vaccines working on the development of vaccines against Respiratory Syncytial Virus (RSV) and human Metapneumovirus (hMPV).
Since Snape’s paper was submitted there have been two further Serious Adverse Events of hMPV disease reported in babies who received the mRNA-1365 vaccine.
Some other children vaccinated in the Moderna trial have likely since been exposed to RSV or hMPV. What has happened to them?
Pride before the Falls
The Moderna babies trial is NOT on the agenda for the upcoming RSV-fest at the 13th International RSV Symposium where in March 2025 you can meet the RSV good and the great at the Iguazu Falls, Brazil. The program has a talk by Ruth Karron about the infamous 1960s Pfizer RSV kids trial which put VAERD on the map. There is nothing about the latest VAERD episode in Panama.
A key scientific issue like VAERD should be on the agenda for a scientific meeting on tackling respiratory infections. We know how to predict VAERD and other respiratory problems but rather than investigate thoroughly and solve our problems, the medico-pharmaceutical complex is hiding them.
You would like the ‘experts’ to discuss these data for admissions to hospitals in England with RSV infections in the young, where roughly half of pregnant mothers got Abrysvo, and for the elderly, where substantial numbers got the vaccine. There is no hint of a benefit compared to prior RSV seasons
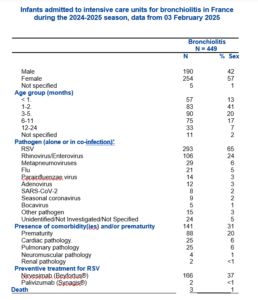
It would also be good to get the experts to embrace these data on admissions to ITUs in France where most infants had been given Beyfortus within days of birth.
Moderna’s mRNA-1345 has been rerouted into clinical trials in pregnant women. Claiming you have solved the problem by creating maternal antibodies that are transferred to the baby via the placenta is the end-run around VAERD that Pfizer and GSK have tried.
Respiratory viruses generate IgA antibodies in lung and gut. Vaccination trials in pregnancy generate IgM antibodies primarily. A mother’s breast generates IgA antibodies and breast-feeding delivers these to babies, without causing hypertension in mothers or preterm births for infants.
It looks like both companies and regulators have a repetition compulsion. They desperately want to be able to say they’ve cracked the VAERD mystery. They are willing to wave Abrysvo through claiming to have solved the problem. Abrysvo has minimal benefits for RSV at a cost of causing serious problems for mother and baby.
I shared my concerns about the animal trials with the 18 experts on the FDA December panel and with the lead authors of the Moderna papers but have had no response from any of them (20 in total).
BMJ mentioned the abandoned trial but declined to accept four “rapid responses” that I sent, pointing out the grounds for concern. Who have BMJ been nobbled by?
This is not a way to solve the problem. We now have expensive minimally effective and perhaps dangerous ‘vaccines’, which may be compromising the effectiveness of other more important vaccines mothers are being told they must have while pregnant.
They may also compromise the effectiveness of vaccines given in early infancy.
They do compromise the natural immunity to hMPV RSV infections give children. RSV, hMPV and other cold viruses are paramyxoviruses – catching one gives you immunity to others. This is why Moderna and other companies are looking at combinations of RSV and hMPV, RSV and flu, and all of them with Covid – because vaccinating against one makes you more vulnerable to more serious infections with the others than you might otherwise have had.
Take Home Messages
- Current RSV vaccines don’t work in a meaningful sense of that word.
- RSV ‘vaccines’ come with a variety of harms
- Semi-mandated RSV “protection” was introduced too soon.
- What will happen to the toddlers “protected” by Abrysvo or Beyfortus who are RSV-naïve when they are later naturally exposed to RSV, or hMPV or other viruses?
- Human and animal data indicate Vaccines cause VAERD.
- Company behaviour – as in Moderna manipulating the data for inflammation in rat lungs (above) and GSK, Pfizer and Sanofi input to this post. Even if we had nothing else to go on, this company behavior close to proves they know their vaccines cause VAERD and/or other serious problems.
- Maternal Vaccine Trials are the Wild West – regulators have no ground rules for running trials or labeling treatments given in the second half of pregnancy.
Magic never Dies
This is a Healy coda to the above post.
Pfizer claimed their maternal RSV vaccine trial showed Abrysvo is over 80% effective. This echoed claims their Covid Vaccine was 95% effective.
These figures sound impressive. But the 95% claim was based on 0.47% of people in the Covid trial. If we let a full human body stand for the 40,000 folk in this trial, the approval was based on One Big Toe’s worth of data. See Dos Centavos but you will have to read down to the end.
The claims are based on statistical lap dancing – to use a phrase from Harriet Vogt’s Rupture post on RxISK – using relative risk data rather than absolute risk data.
Our Big Toe idea didn’t go viral. Perhaps the mysterious image that opens this post will.
Reworking the relative risk 80+% claim for Abrysvo into absolute risk terms, we end up with Abrysmo protecting a little over half of I in 100 babies from a relatively minor infection.
Dos Centavos shows you a whole human body and its toe. How do we show you half of a baby – saw one in half?
We needed the help of a magician. Here’s hoping this bit of magic can counteract Pfizer’s magic trick which unfortunately does result in harmed or even dead babies.
The post The Respiratory Syncytial Virus Challenge first appeared on Dr. David Healy.