October 09, 2025
3 min read
Key takeaways:
- Surgery and stereotactic ablative radiotherapy conferred comparable 10-year survival among patients with early-stage NSCLC.
- Fewer complications occurred in the radiotherapy group.
A long-term analysis of patients with early-stage non-small cell lung cancer showed those who received radiation achieved survival comparable to those who underwent surgery.
Individuals who received stereotactic ablative radiotherapy (SABR) reported fewer short-term complications than those who had lobectomy, results of the prospective STARS trial showed.

Data derived from Kleber T, et al. Abstract 268. Presented at: ASTRO Annual Meeting; Sept. 27-Oct. 1, 2025; San Francisco.

Joe Y. Chang
The findings — presented at American Society for Radiation Oncology Annual Meeting — demonstrate stereotactic radiotherapy is “a strong alternative to surgery” for the majority of patients with stage I NSCLC who are eligible for surgery, according to Joe Y. Chang, MD, PhD, FASTRO, professor of thoracic radiation oncology and director of stereotactic ablative radiotherapy at The University of Texas MD Anderson Cancer Center.
Surgery had historically been standard for early-stage NSCLC. However, up to half of patients may experience moderate to severe complications, according to study background. Also, the median age of lung cancer diagnosis in the U.S. is 71 years, and older patients may not be able to tolerate surgery.
Consequently, a noninvasive approach that can offer durable local control is needed, Chang said.
Patients with early-stage NSCLC who are unable to undergo surgery often receive SABR, in which high doses of radiation are delivered over five or fewer sessions.
Some evidence suggests SABR could confer similar survival benefits as surgery even among patients who are eligible to undergo resection.
Chang and colleagues conducted the phase 2 STARS trial to prospectively compare long-term outcomes with the two approaches among patients treated at MD Anderson Cancer Center between 2015 and 2017.
Eighty patients who had tumors smaller than 3 cm, no distant metastases and no lymph node involvement received SABR in three or four sessions.
Researchers used an institutional dataset to establish a surgery cohort matched for age, sex, tumor size and health status. Those 80 patients underwent video-assisted thoracoscopic lobectomy with mediastinal lymph node dissection.
Investigators followed patients for up to 10 years.
OS served as the primary endpoint. Other outcomes included adverse events, recurrence, quality of life and financial impact of treatment.
Median follow-up reached 8.3 years.
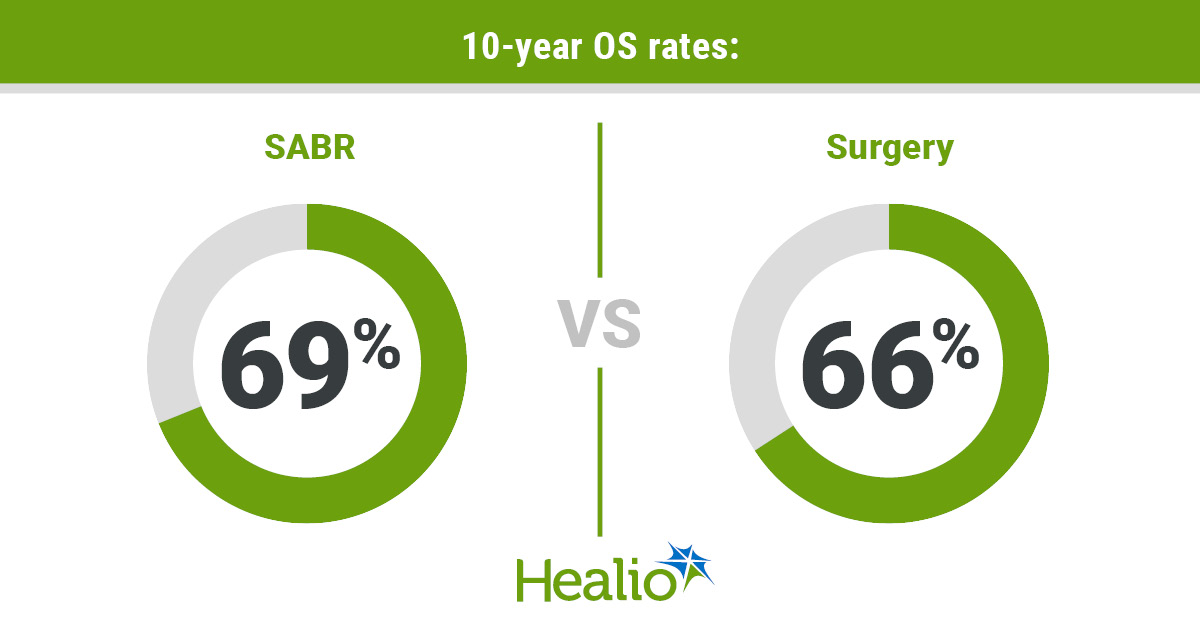
Researchers reported comparable rates of 10-year OS in the SABR and surgery groups (69% vs. 66%; HR = 0.77; 95% CI, 0.42-1.44). Results also showed similar rates of lung-cancer specific survival (92% vs. 89%; HR = 0.95; 95% CI, 0.31-2.94) and recurrence-free survival (57% vs. 65%; HR = 1.17; 95% CI, 0.67-2.04).
“Since oncologic outcomes were comparable, the next question we asked was: How about quality of life,” Chang said during a press conference.
Half (50%) of patients who underwent surgery developed grade 3 or higher short-term lung or cardiovascular complications, compared with 1% of those who received SABR.
Surveys of patients who survived 10 years showed comparable physical and mental quality of life between treatment groups, with no significant difference in financial burden.
Up to one-third of patients treated with local therapies develop locoregional or distant recurrence, so patients who undergo SABR must be monitored closely, Chang said.
“AI-guided image biomarker-based personalized SABR, with or without immunotherapy or targeted therapy, is expected to further improve clinical outcomes and inform future research directions,” Chang said.