August 05, 2025
4 min read
Key takeaways:
- Canagliflozin was tied to a greater HbA1c decrease than placebo among children with type 2 diabetes.
- There were no serious adverse events related to the study drug.
Children and adolescents with type 2 diabetes had greater reductions in HbA1c with an oral SGLT2 inhibitor compared with placebo, researchers reported.
In December, the FDA approved an expanded indication for canagliflozin (Invokana, Janssen) to include children and adolescents aged 10 to 17 years with type 2 diabetes based on findings from a phase 3 randomized controlled trial. The trial, now published in Annals of Internal Medicine, showed youths with type 2 diabetes receiving canagliflozin had a greater HbA1c decline from baseline to 26 weeks compared with those receiving placebo.

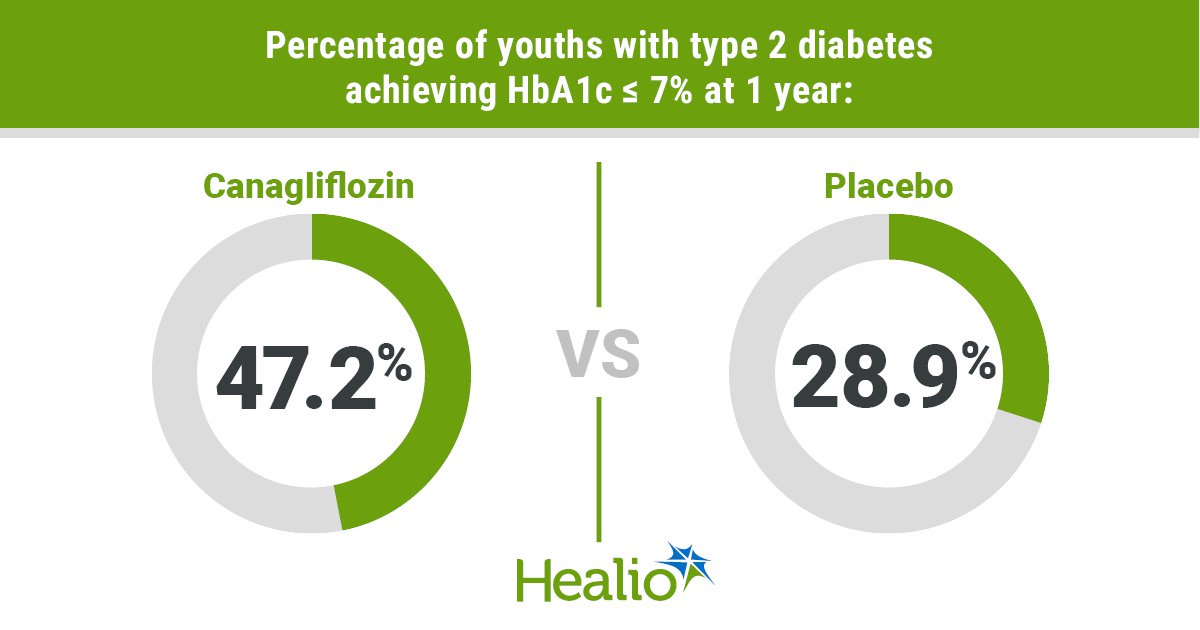
Data were derived from Nadgir U, et al. Ann Intern Med. 2025;doi:10.7326/ANNALS-24-04017.
“The results of this study showed that canagliflozin was safe and well tolerated, and provides a significant and clinically meaningful reduction in HbA1c compared with placebo in children and adolescents aged 10 to 17 years with type 2 diabetes, which was observed early at week 6 and was sustained until the end of the treatment period,” Saberi Rana Ali, MD, MPH, clinical leader at Johnson & Johnson Innovative Medicine, and colleagues wrote. “These results were also confirmed in the subgroup of participants who received metformin with or without insulin.”
Researchers enrolled 171 children and adolescents aged 10 to 17 years with type 2 diabetes and an HbA1c of 6.5% to 11% who partook in diet and exercise intervention prior to the study for at least 4 weeks with or without metformin. Following a 2-week run-in period, participants were randomly assigned to receive once-daily 100 mg oral canagliflozin (n = 84) or placebo (n = 87). At 13 weeks, 33 youths receiving canagliflozin who still had an HbA1c of 7% or higher and an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or greater were randomly assigned to continue 100 mg of canagliflozin (n= 16), increase their daily dose of canagliflozin to 300 mg (n = 17). The trial continued for 1 year. The primary outcomes were change in HbA1c from baseline to 26 weeks and safety and tolerability.
HbA1c change
At 26 weeks, youths receiving canagliflozin had a greater HbA1c decline than those receiving placebo (least-squares mean difference, –0.76 percentage points; 95% CI, –1.25 to –0.27; P = .002). Findings were similar when the analysis was limited to those who also received metformin, with the canagliflozin group having a greater decline in HbA1c than the placebo group (least-squares mean difference, –0.77 percentage points; 95% CI, –1.38 to –0.17; P = .012).
Canagliflozin was associated with a greater decline in fasting plasma glucose than placebo at 26 weeks (least-squares mean difference, –25.5 mg/dL; 95% CI, –49.55 to –1.47) and 52 weeks (least-squares mean difference, –27.8 mg/dL; 95% CI, –46.4 to –9.15).
At 26 weeks, the canagliflozin group was more likely to have an HbA1c of less than 6.5% (36.3% vs. 14%) or less than 7% (45.7% vs. 32.8%) compared with placebo. The same was true at 1 year (HbA1c less than 6.5%: canagliflozin,30.9%; placebo, 15.6%; HbA1c less than 7%: canagliflozin, 47.2%; placebo, 28.9%). At 1 year, rescue medication was required for 46% of the placebo group compared with 11.9% of the canagliflozin group. Children and adolescents receiving canagliflozin had a 1.6 percentage point greater decline in body weight vs. the placebo group.
The researchers wrote canagliflozin provides cardiovascular and renal benefits for adults with type 2 diabetes and while those outcomes have not been assessed among children, it is possible the therapy could reduce the long-term risk for several cardiometabolic diseases.
“A treatment intervention that provides robust glycemic control and a potential reduction in the risk for long-term cardiorenal complications would be an important addition to the treatment options for children and adolescents with type 2 diabetes,” the researchers wrote.
Safety data
Treatment-emergent adverse events occurred in 77.4% of the canagliflozin group and 74.7% of the placebo group. The canagliflozin group had a higher percentage of participants reporting headaches (10.7% vs. 3.4%), nasopharyngitis (9.5% vs. 5.7%), urinary tract infection (7.1% vs. 4.6%) and vomiting (6% vs. 2.3%).
Serious adverse events were reported by 9.5% of the canagliflozin group and 5.7% of the placebo group. There was one case of diabetic ketoacidosis, one pancreatitis event and one report of fracture in the canagliflozin group. No serious adverse events were related to canagliflozin.
Symptomatic hypoglycemia was reported by 11.9% of the canagliflozin group and 10.3% of the placebo group. Hypoglycemia with a glucose of less than 70 mg/dL occurred among 17.9% of the canagliflozin group and 16.1% of the placebo group, and hypoglycemia with a glucose below 56 mg/dL occurred among six children receiving 100 mg canagliflozin and seven receiving placebo. One severe hypoglycemia case occurred in the placebo group.
‘An important addition’
In a related editorial, Ryan P. Brady, MD, MS, assistant professor of pediatrics at University of Cincinnati College of Medicine and in the division of endocrinology at Cincinnati Children’s Hospital Medical Center; and Amy S. Shah, MD, MS, FNLA, director of the division of endocrinology at Cincinnati Children’s Hospital Medical Center, wrote the findings of the trial were similar to prior studies assessing other SGLT2 inhibitors in children and adolescents with type 2 diabetes, and that the effects of oral canagliflozin were similar to those observed with GLP-1s.
Brady said the rising prevalence of type 2 diabetes among children and adolescents makes finding new treatment options imperative.
“Canagliflozin has shown both safety and efficacy for HbA1c lowering in youth-onset type 2 diabetes, making it an important addition to the therapeutic options available in this growing population,” Brady told Healio.
Brady and Shaw wrote in their editorial that the “aggressive nature of youth-onset type 2 diabetes means that combination medical therapy is often necessary.” Brady told Healio that further research into combination therapies is crucial due to high treatment failure rates among children and adolescents with type 2 diabetes.
“Given the early onset and nature of youth-onset type 2 diabetes, future studies evaluating the effect of SGLT2 inhibitors on disease progression are important,” Brady said.
References:
Brady RP, et al. Ann Intern Med. 2025;doi:10.7326/ANNALS-25-02805.
Invokana (canagliflozin) tablets. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/204042Orig1s043;204353Orig1s046;205879Orig1s023ltr.pdf. Published Dec. 18, 2024. Accessed Aug. 4, 2025.
For more information:
Ryan Brady, MD, MS, can be reached at endocrinology@healio.com.