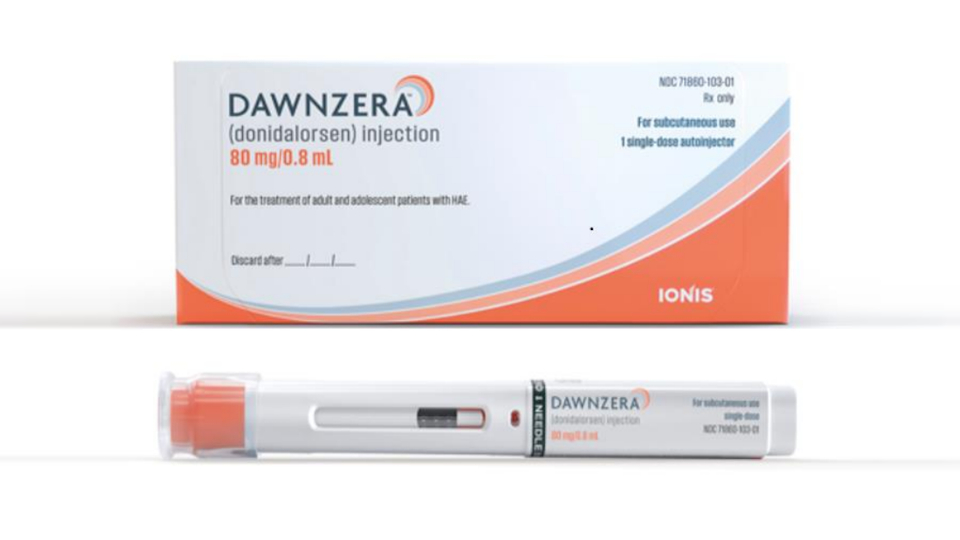
Ionis has won an FDA green light for an RNA-based hereditary angioedema (HAE) treatment, Dawnzera, that can be self-administered by patients every four or eight weeks.
The first-in-class drug targets plasma prekallikrein (PKK), a protein that activates inflammatory mediators associated with HAE attacks, unpredictable bouts of debilitating and painful swelling around the body that afflict patients with the rare genetic disorder.
Its dosing offers the longest intervals between injections among newer drugs to prevent HAE attacks, which include CSL’s recently approved Factor XIIa inhibitor Andembry (garadacimab), dosed monthly, and Takeda’s blockbuster kallikrein inhibitor Takhzyro (lanadelumab), which requires injections every two weeks.
Ionis said that Dawnzera (donidalorsen) will be launched in the US in the coming days and will have a list price of $57,462 per dose. HAE is estimated to affect about 7,000 patients in the US, according to the company, of whom around three-quarters need prophylactic treatment.
It is the Californian biotech’s second launch of a non-partnered product in nine months, coming after it introduced Tryngolza (olezarsen) in the US as the first-ever therapy for familial chylomicronaemia syndrome (FCS), and is one of four independent products that Ionis intends to bring to market by the end of 2026.
The others are olezarsen for severe hypertriglyceridaemia (sHTG), due for two phase 3 trial readouts next month, and zilganersen, in phase 3 for the rare and fatal neurological condition Alexander disease (AxD).
There is a “multibillion-dollar sales opportunity” across these four launches, according to Ionis, which has been working to build its own commercial operations and reduce its reliance on partnering.
Dawnzera’s approval is based on the results of the placebo-controlled OASIS-HAE study, in which a monthly dose of the drug reduced the monthly HAE attack rate by 81% over 24 weeks, with similar efficacy seen with eight-weekly dosing.
Analyst Yaron Werber at TD Cowen has predicted that annual sales of Dawnzera could top $500 million by 2032, according to Reuters, pointing to an appetite for switching to the new drug among patients who are not doing well on current therapies.
Ionis chief executive Brett Monia said that Dawnzera represents “a significant advance for people living with HAE who need improved treatment options,” pointing out that around 20% of HAE patients switch treatments annually.
“With strong and durable efficacy, convenient administration and the longest dosing option available, we believe Dawnzera will be the prophylactic treatment of choice for many people living with HAE.”