Shots:
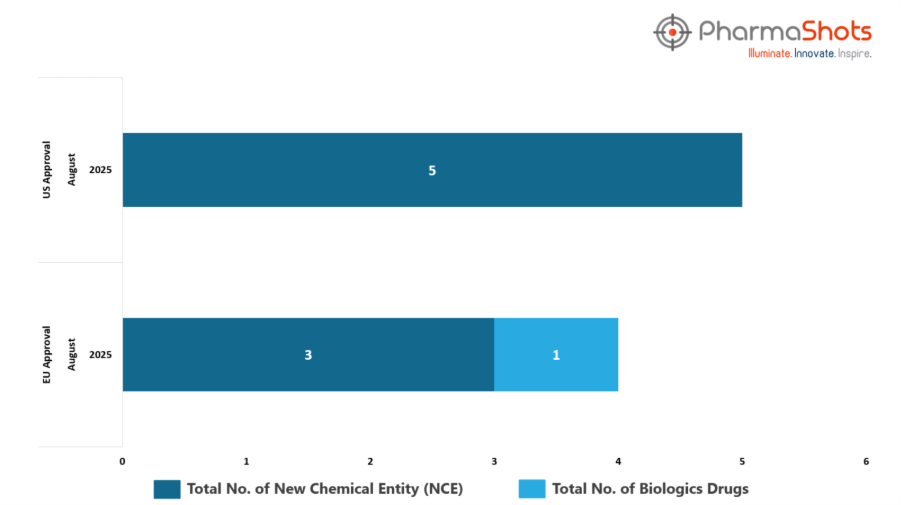
- The EMA’s CHMP has granted approvals to 1 Biologic and 3 new chemical entities in August 2025, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drug was Gilead’s Yeytuo has Secured the EC’s Approval for Pre-Exposure Prophylaxis (PrEP) to Prevent HIV
- PharmaShots has compiled a list of 4 drugs that have been granted approvals by the EC
Company: SpringWorks Therapeutics (Merck KGaA)
Product: Ogsiveo
Active Ingredient: Nirogacestat
Disease: Desmoid Tumors
Date: Aug 18, 2025
Shots:
- The EC has approved Ogsiveo as a monotx. for adults with progressing desmoid tumors needing systemic treatment
- Approval was based on global P-III (DeFi) trial (N=142) assessing Nirogacestat (150mg, BID, n=70) vs PBO (n=72) in adults with progressing desmoid tumors
- Trial met its 1EP with 71% reduction in risk of disease progression & showed improved ORR (41% vs 8%), CR (7% vs 0%), median time to first response (5.6 vs 11.1mos.) & PROs, incl. pain, desmoid tumor-specific symptoms, physical/role functioning, & overall health-related QoL; published in the NEJM
Company: Madrigal Pharmaceuticals
Product: Rezdiffra
Active Ingredient: Resmetirom
Disease: Noncirrhotic metabolic Dysfunction-associated Steatohepatitis with Mod. To Adv. Liver Fibrosis
Date: Aug 19, 2025
Shots:
- The EC has granted conditional approval to Rezdiffra to treat adults with noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH) with mod. to adv. liver fibrosis in 30 EEA states; launch is planned throughout EU starting with Germany in Q4’25
- Approval was based on P-III (MAESTRO-NASH) trial assessing Rezdiffra (100 & 80mg, PO, QD) vs PBO in MASH pts, which met its 1EPs of fibrosis improvement & MASH resolution, plus showed reduced liver stiffness, liver fat, liver enzymes, & atherogenic lipids, with 91% pts on 100mg showing improvement or stabilization in liver stiffness
- Rezdiffra is being evaluated in a fully enrolled, ongoing P-III (MAESTRO-NASH OUTCOMES) trial against PBO to assess its impact on liver decompensation in MASH cirrhosis
Company: Gilead
Product: Yeytuo
Active Ingredient: Lenacapavir
Disease: Pre-Exposure Prophylaxis (PrEP) to Prevent HIV
Date: Aug 26, 2025
Shots:
- Approval was based on P-III (PURPOSE 1 & PURPOSE 2) trials of Yeytuo vs Truvada, where PURPOSE 1 showed 0 infections & 100% risk reduction in 2134 women, while PURPOSE 2 depicted 99.9% non-infection rate in 2179 pts (2 acquired HIV), showing superiority to bHIV in both trials; published in The NEJM
- Approval is valid in all 30 EEA states, with regulatory review ongoing in Australia, Brazil, Canada, South Africa as well as Switzerland & filing is underway in Argentina, Mexico & Peru
- Yeytuo has obtained an additional yr. of EU market protection for significant clinical benefit &, following an EU-M4all positive opinion for PrEP, Gilead plans accelerated LMIC filings, prioritizing 18 countries covering 70% of HIV burden
Company: ExCellThera
Product: Zemcelpro
Active Ingredient: Allogeneic Umbilical CD34- Cells, Non-expanded / Dorocubicel
Disease: Haematological Malignancies
Date: Aug 27, 2025
Shots:
- The EC has granted conditional approval to Zemcelpro in all 30 EEA states for adults with haematological malignancies needing ASCT after myeloablative conditioning, when no other suitable donor cells are available; regulatory filing is underway in the US, UK, Canada & Switzerland
- Zemcelpro availability will vary by country based on national reimbursement processes; meanwhile, Cordex Biologics (ExCellThera’s subsidiary) is collaborating with health authorities for early patient access, building a treatment center network, & pursuing strategic partnerships to drive commercialization in Europe & other regions
- Zemcelpro (UM171 Cell Therapy) is a personalized cryopreserved stem cell transplantation product comprising UM171-expanded CD34+ cells (dorocubicel) & unexpanded CD34− cells, both derived from the same cord blood unit; P-III trial for above pts to be initiated soon
Related Post: Insights+: EMA Marketing Authorization of New Drugs in July 2025










