August 14, 2025
2 min read
Key takeaways:
- Adults who received dupilumab achieved higher complete response rates for cancer therapy–related skin toxicities than those who received steroids.
- Dupilumab offers patients a steroid-sparing option.
Dupilumab may provide a nonsteroidal option for managing cancer therapy-related skin toxicities, according to a study.
Treatment with antibody-drug conjugates (ADCs) for malignant neoplasms is known to cause dermatologic adverse events, including eczematous, morbilliform and vesiculobullous eruptions, sometimes leading patients to discontinue their cancer treatment. Traditionally, dermatologists prescribed systemic steroids to mitigate these adverse events, but the use of steroids raises concerns.

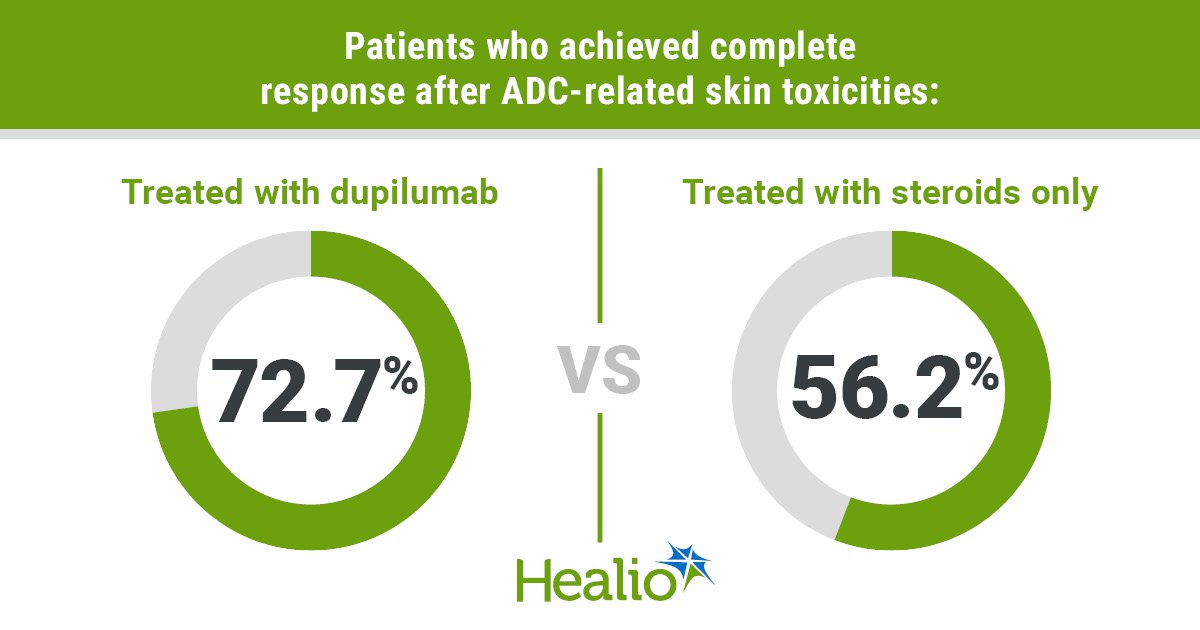
Data derived from Nykaza I, et al. JAMA Dermatol. 2025;doi:10.1001/jamadermatol.2025.2567.
“With steroids, there is often an immune suppression and other side effects that are intolerable,” Allison Gordon, MD, director of outpatient oncodermatology at Memorial Sloan Kettering Cancer Center, told Healio. “Generally speaking, if we were to give people steroids, it might counteract some of the benefit that they are getting from their anticancer therapy.”

Allison Gordon
A recent publication in JAMA Dermatology highlights the possibility of treating these adverse dermatologic events with dupilumab (Dupixent; Sanofi, Regeneron), an interleukin-4 receptor alpha antagonist and steroid-sparing agent, instead of steroid therapy.
Gordon and colleagues evaluated the outcomes of 27 participants with ADC-induced cutaneous toxicities, according to records from Memorial Sloan Kettering Cancer Center. Eleven of these patients were treated with dupilumab 600 mg as a loading dose, followed by 300 mg biweekly, and 16 received systemic steroids only.
Results showed that 72.7% of participants treated with dupilumab achieved a complete response compared with 56.2% of those treated with steroids only. Additionally, 27.3% in the dupilumab group and 25% in the steroid-only group achieved a partial response. In the steroid group, three participants were deemed nonresponders vs. zero in the dupilumab group.
Treatment discontinuation due to dermatologic adverse events was 43.8% less in the dupilumab vs. the steroid-only group (0 vs. 7; P < .05).
According to Gordon, these findings show that dupilumab may allow patients to remain on their ADC cancer treatment for a longer period.
“If we hadn’t given patients dupilumab, we might have been quicker to say to the oncology teams that they weren’t able to safely give this anticancer drug, and that the side effects were causing a quality-of-life impact that was too dire to continue,” Gordon told Healio. “With dupilumab, these patients were able to receive the benefit of life-prolonging therapy.”
For more information:
Allison Gordon, MD, can be reached at gordona3@Mskcc.org.