August 26, 2025
4 min read
Key takeaways:
- Children and adults had an improvement in time in range with the MiniMed 780G insulin pump and Simplera Sync sensor.
- Time in range also improved for those using the system’s recommended optimal settings.
Use of an automated insulin delivery system that combines an insulin pump with a disposable sensor improved time in range and lowered HbA1c for children and adults with type 1 diabetes, according to data from the SUCCEED study.
In an open-label single-arm trial, children and adults with type 1 diabetes used the MiniMed 780G insulin pump (Medtronic) along with the Simplera Sync disposable continuous glucose monitoring sensor. According to a press release from Medtronic, the FDA approved the use of Simplera Sync with the MiniMed 780G in April. As Healio previously reported, the FDA approved the MiniMed 780G for children and adults with type 1 diabetes in April 2023.

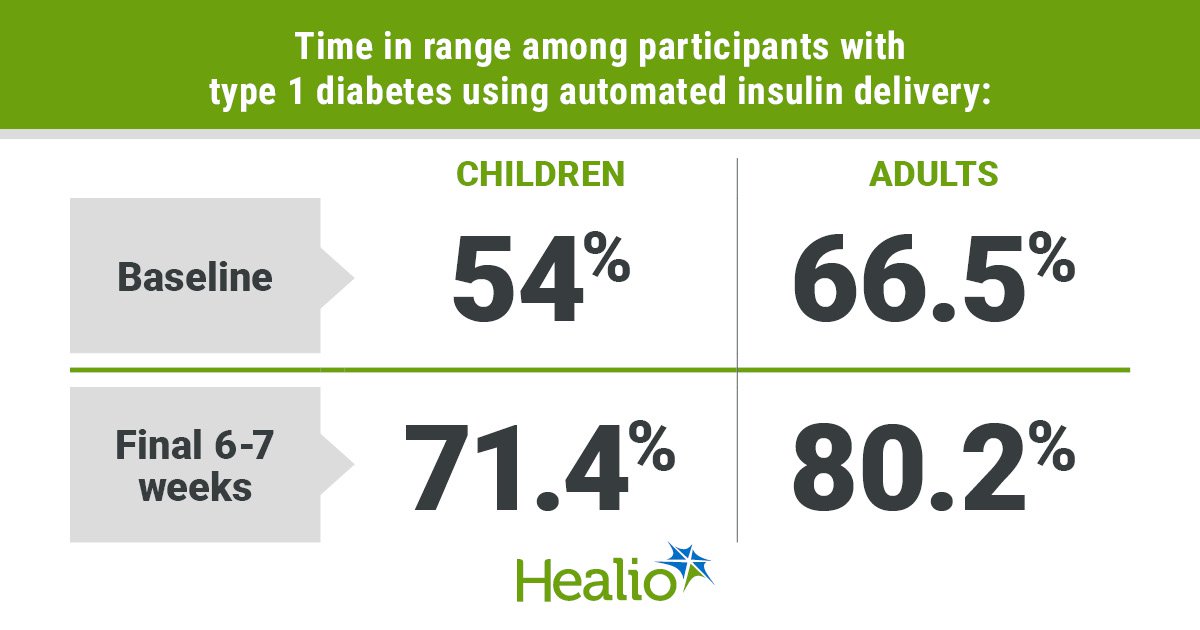
Data were derived from Nally LM, et al. Diabetes Technol Ther. 2025;doi:10.1177/15209156251368928.
Laura M. Nally, MD, assistant professor of pediatrics at Yale School of Medicine, said the addition of the sensor helps simplify diabetes management in several ways.

Laura M. Nally
“The Simplera Sync sensor is smaller and lighter than previous versions, does not require finger-stick calibrations, uses a simplified two-step process for insertion and features a disposable sensor with an integrated transmitter, eliminating the need for a separate transmitter that requires charging,” Nally told Healio. “Consequently, this reduces the time commitment and mental strain for individuals living with type 1 diabetes.”
Researchers enrolled children and adults aged 7 to 80 years with type 1 diabetes who required at least 8 U insulin daily, had an HbA1c of less than 10% at screening and used an insulin pump for more than 6 months before the study. The study began with a 2-week run-in period where participants were educated on using both the insulin pump and sensor. The system was used as a sensor-augmented pump during the run-in period. After the run-in period, participants enabled the auto basal and auto correction on the system and used it for approximately 3 months. The primary effectiveness endpoint was mean time in range with glucose between 70 mg/dL and 180 mg/dL during the last 6 to 7 weeks of the study. The primary safety endpoint was change in HbA1c from baseline to the end of the study.
The study group included 107 children and adolescents aged 7 to 17 years and 105 adults aged 18 to 80 years. The system was active more than 93% of the time for children and adolescents and more than 96% of the time for adults.
Glycemic outcomes
Children and adolescents had an increase in mean time in range from 54% at baseline to 71.4% in the study’s final 6 to 7 weeks (P < .001). Similarly, mean time in range for adults rose from 66.5% at baseline to 80.2% in the final 6 to 7 weeks of the study (P < .001). Nally said both children and adults exceeded consensus guideline recommendations of 70% or higher time in range by the end of the study.
“This is especially important for children with type 1 diabetes, as remembering to enter carbohydrates and give correction doses is challenging, and more than 70% time in range is very difficult to achieve in this population,” Nally said.
Youths had an increase in time below range from 1.6% at baseline to 1.9% in the last 6 to 7 weeks of the study (P < .001). No change in time below range was seen for adults.
Mean sensor glucose decreased from 180.4 mg/dL during the run-in period to 154.4 mg/dL at the end of the study for youths. Adults had a decline in mean sensor glucose from 161 mg/dL during the run-in period to 142.2 mg/dL with automated insulin delivery.
The proportion with an HbA1c less than 7% rose from 19.6% to 37.6% in children and adolescents and from 30.9% to 69.2% in adults.
Children and adolescents had 41 U automated basal and correction insulin delivered daily by the system, which was equivalent to about 65% of their total daily insulin dose. Adults received 36.5 U automated basal and correction insulin daily, for about 61% of their total daily insulin.
Of the participants, 41 youths and 44 adults used the system’s recommended optimal setting of a 100 mg/dL glucose target with a 2-hour active insulin time. Among the subgroup, time in range was 74.7% for children and 83.8% for adults at the end of the study. The percentage of participants using optimal settings who achieved an HbA1c less than 7% at the end of the study was 46.3% among children and adolescents and 81% among adults.
“The 100 mg/dL glucose target is one of the lowest glucose targets of commercially available automated insulin delivery systems in the U.S., giving people with type 1 diabetes the necessary tools and functionality to spend more time in range,” Nally said. “The algorithm was really driving these increases in time in range, providing 60% of the total daily insulin dose.”
Safety data
Children had a reduction in mean HbA1c from 7.7% at baseline to 7.3% with use of the automated insulin delivery system. Mean HbA1c for adults declined from 7.4% at baseline to 6.7% with automated insulin delivery.
No serious adverse events occurred among the pediatric group. For adults, there were three serious adverse events among adults, two cases of severe hypoglycemia and one diabetic ketoacidosis event, none of which were related to the study system.
Reference:
New Simplera Sync sensor for the MiniMed 780G system now FDA approved. https://news.medtronic.com/2025-04-18-New-Simplera-Sync-TM-sensor-for-the-MiniMed-TM-780G-System-now-FDA-approved. Published April 18, 2025. Accessed Aug. 22, 2025.
For more information:
Laura M. Nally, MD, can be reached at endocrinology@healio.com.