August 04, 2025
2 min read
Key takeaways:
- Adults with OSA and type 2 diabetes prescribed tirzepatide vs. liraglutide or semaglutide had a smaller composite major adverse cardiovascular event (MACE) incidence rate.
- The MACE outcome included four events.
The risk for major adverse cardiovascular events decreased with tirzepatide vs. liraglutide and semaglutide in patients with obstructive sleep apnea and type 2 diabetes, according to data published in Annals of the American Thoracic Society.
“These findings have the potential to change the short-term symptoms of OSA while impacting the significant long-term cardiovascular consequences in these high-risk cases,” Alex E. Henney, MBChB, MRes, academic clinical fellow at University of Liverpool, and colleagues wrote.

Data were derived from Henney AE, et al. Ann Am Thorac Soc. 2025;doi:10.1513/AnnalsATS.202409-923OC.
In a retrospective cohort analysis, Henney and colleagues evaluated adults with OSA and type 2 diabetes from TriNetX to determine how the risk for incident major adverse cardiovascular events (MACEs) — acute coronary syndrome, heart failure, cerebrovascular accident, and sudden cardiac death — differs with the GIP/GLP-1 dual agonist tirzepatide (Mounjaro/Zepbound, Eli Lilly) vs. GLP-1 receptor agonists liraglutide (Victoza/Saxenda, Novo Nordisk) and semaglutide (Ozempic/Wegovy, Novo Nordisk) for 18 months.
When comparing patients treated with tirzepatide vs. patients treated with liraglutide, researchers reported 7,836 patients in each group after propensity score matching that factored in age, sex, ethnicity, smoking, socioeconomic status, hypertension, dyslipidemia, BMI, glomerular filtration rate, HbA1c, other blood glucose-lowering therapies and corticosteroids.
The analysis comparing patients prescribed tirzepatide vs. semaglutide included 7,394 patients in each group following propensity score matching.
Tirzepatide vs. liraglutide
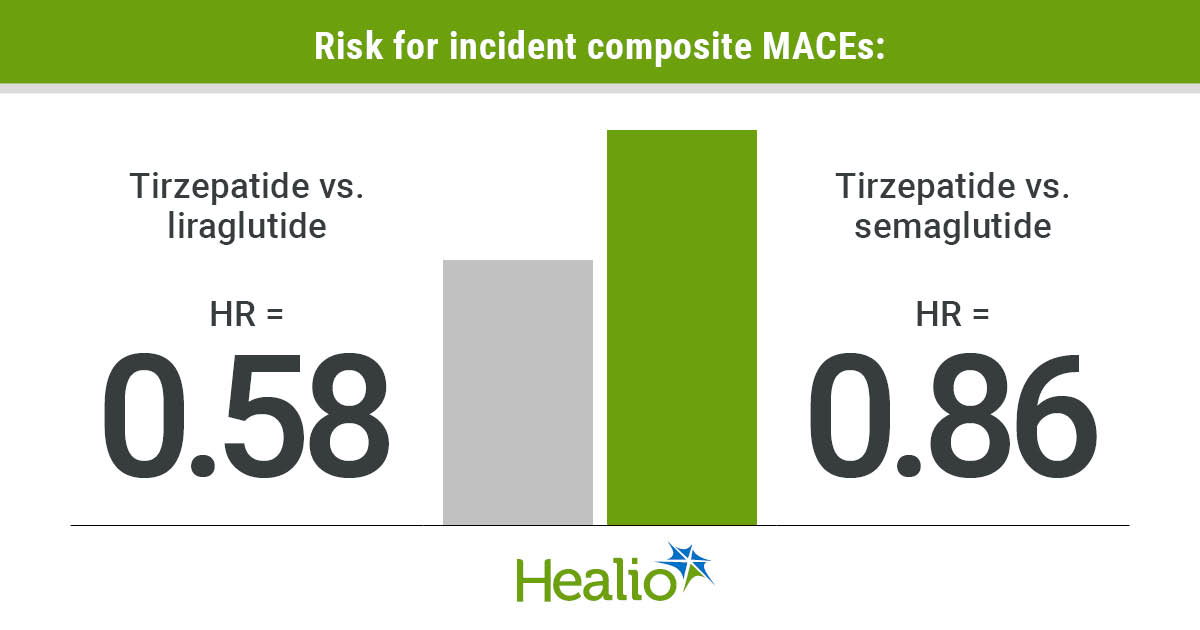
Between tirzepatide and liraglutide, the risk for incident composite MACEs was significantly lower with tirzepatide (HR = 0.58; 95% CI, 0.51-0.66). When broken down into the four MACEs that made up the composite outcome, the study reported a significantly decreased risk among patients prescribed tirzepatide vs. liraglutide for acute coronary syndrome (HR = 0.48; 95% CI, 0.33-0.72), cerebrovascular accident (HR = 0.56; 95% CI, 0.44-0.71) and heart failure (HR = 0.48; 95% CI, 0.4-0.58).
Aligning with the observed lower risk for incident MACEs, researchers found a smaller composite MACE incidence rate with tirzepatide vs. liraglutide (28.9 vs. 54.6).
Notably, the significantly lower risk for incident MACEs with tirzepatide vs. liraglutide was reported across several subgroups. According to the study, this outcome was found in patients with and without obesity; younger (< 60 years) and older (≥ 60 years) adults; men and women; and white and non-white patients. Researchers also found this outcome regardless of study drug adherence and geographical location.
Tirzepatide vs. semaglutide
Similar to above, the risk for incident composite MACEs was significantly decreased with tirzepatide vs. semaglutide (HR = 0.86; 95% CI, 0.74-0.99), and the composite MACE incidence rate was smaller with tirzepatide (27.6 vs. 36), according to the study.
In this set of patients, researchers noted that the only individual MACE that tirzepatide significantly lowered the risk for vs. semaglutide was cerebrovascular accident (HR = 0.74; 95% CI, 0.55-0.97).
In contrast to the tirzepatide vs. liraglutide analysis, the significantly lower risk for incident MACEs with tirzepatide vs. semaglutide was reported in select patients. According to the study, this outcome was found in patients with obesity (HR = 0.81; 95% CI, 0.67-0.97), younger adults (HR = 0.72; 95% CI, 0.58-0.91), white patients (HR = 0.81; 95% CI, 0.69-0.96) and patients adherent to the study drug (HR = 0.81; 95% CI, 0.67-0.98).
“Given the nature of our methods with real-world, retrospective data and the inherent limitations, our findings may be considered hypothesis-generating, with comparative randomized controlled clinical trials required to provide confirmatory evidence of our findings,” Henney and colleagues wrote.