As pharmaceutical manufacturing reindustrializes, plants are under pressure to turn vast, fragmented data into timely, compliant decisions on the shop floor. Supported by Tier Collaboration Dashboards (Tier Boards) and the built-in SAMI (Shiftconnector® Artificial Manufacturing Intelligence) assistant, eschbach’s Shiftconnector® addresses this need by unifying operational data with the context teams rely on every shift. This focus on real-time clarity and structured collaboration earned the company two awards in the 2025 Pharmaceutical Technology Excellence Awards.
eschbach won the Innovation award for Operational Intelligence for consolidating real-time plant data and context into role-specific visual dashboards that support faster, compliant decisions. The company also won the Product Launches award for integrating an artificial intelligence (AI) assistant into daily workflows to help teams analyze issues, share knowledge, and act on insights within a shared operational workspace.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Operational intelligence in Pharma: Unified data and role-specific visualization
What set eschbach apart in operational intelligence is the way it treats plant information as a daily management system rather than a collection of point solutions. Shiftconnector® pulls structured data from MES, LIMS, SCADA, ERP, and data historians into a centralized operational view, and it also captures the unstructured context that often explains why events happen—shift handover notes, operator logs, deviation records, and near-miss reports. That combination addresses a familiar pain point in pharma plants: data exists in abundance, but the narrative connecting it to real operations is scattered.
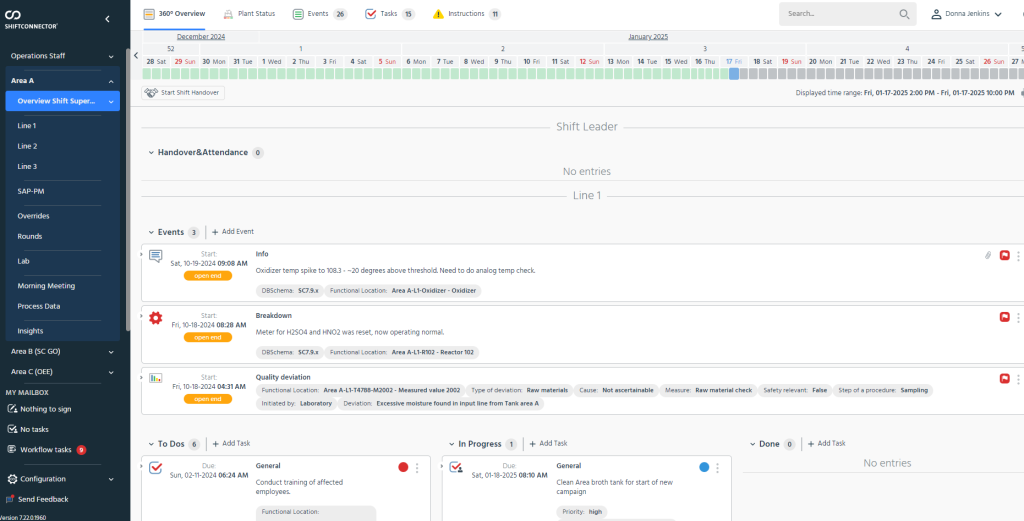
The platform’s Tier Boards translate that unified data into role-specific visualizations. Frontline operators can see current batch status, deviations, alarms, and immediate priorities at a glance. Supervisors access trends, escalation status, and resource constraints. Site leaders view cross-line performance, quality KPIs, and safety indicators. The dashboards are not static; configurable widgets allow users to drill into anomalies and click through to underlying Shiftconnector® entries, including timestamps, notes, corrective actions, and historical references. This linkage shortens the path from signal to root cause while preserving traceability aligned with good manufacturing practice (GMP) and data integrity expectations.
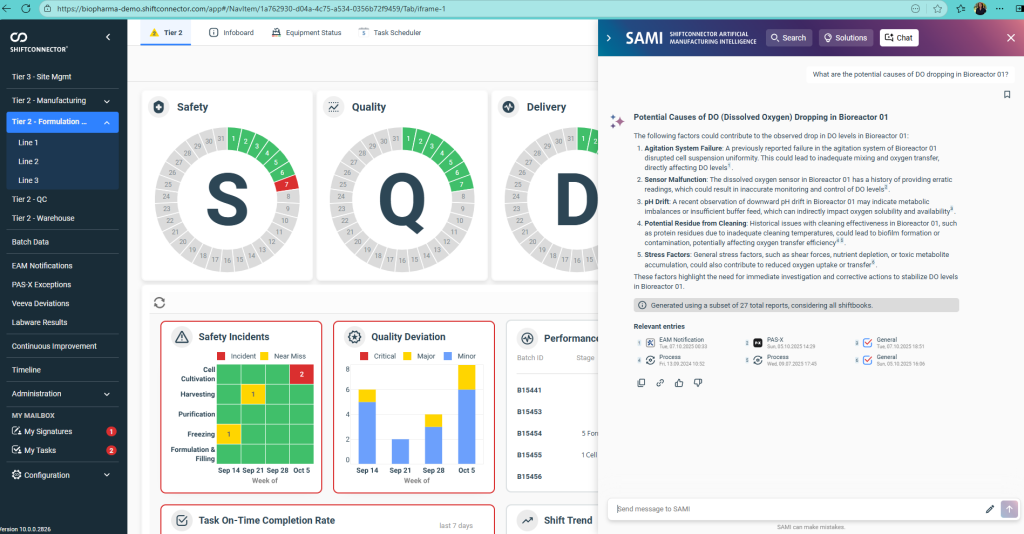
Standardization is another differentiator. The system supports consistent taxonomy and KPI definitions across lines and sites, which is important for organizations with heterogeneous system landscapes or legacy differences. Escalation protocols are embedded so that issues are routed and resolved in a structured way, and audit trails and role-based access keep records complete and permissions clear. These features together help teams move from reactive, after-the-fact reporting to earlier detection and more deliberate, compliant decision-making during the shift.
Importantly, the design accommodates site-specific processes and terminology. Plants can configure thresholds and alerts according to their production realities without losing comparability at the enterprise level. This balance between standardization and flexibility is central to operating in regulated environments where no two facilities are identical. The result is real-time, contextual and actionable view, allowing teams to focus on the right issues quickly, reduce downtime, and maintain alignment across shifts and departments.
Context-aware analytics embedded in tiered workflows
eschbach’s Product Launch recognition centers on SAMI, the AI assistant integrated directly into Shiftconnector®’s Tier Boards. Rather than functioning as a separate analytical layer, SAMI is placed within the same interface teams use for morning meetings, shift handovers, and incident reviews. In practice, this means analysis happens where decisions are made, and insights are immediately tied to relevant event entries and operational data.
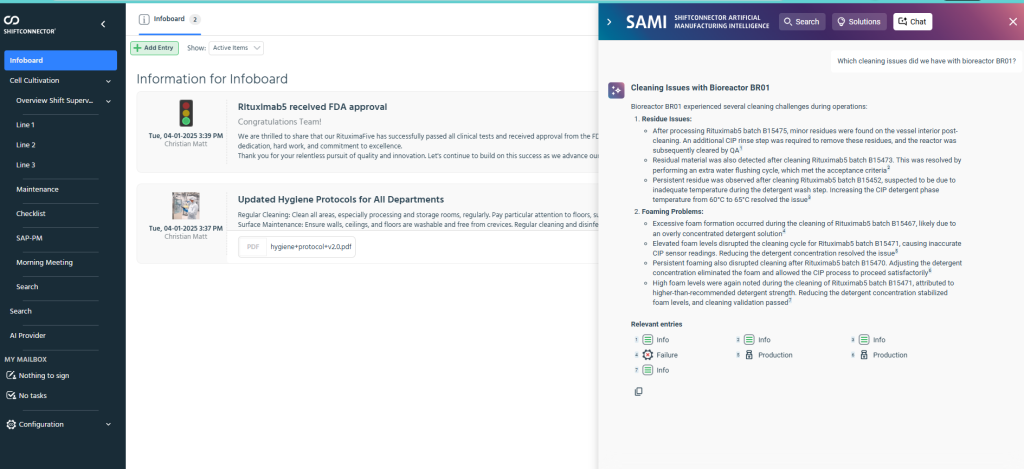
SAMI introduces three capabilities that are particularly relevant to pharma operations. First, Smart Search enables users to pose questions in plain language and retrieve pertinent records—such as deviations, event logs, and maintenance notes—reducing time spent searching across multiple systems. Second, Smart Solutions analyzes historical data to suggest likely root causes and previously applied corrective actions, helping teams resolve issues faster and reinforce proven fixes. Third, Chat with SAMI provides a conversational way to explore trends, drill into contributing factors, and follow up with clarifying questions, all within the dashboard.
The effect is to put context-aware analytics in the hands of different roles, not just specialists. A shift supervisor can spot a deviation on the Tier Board, ask SAMI for similar past incidents, and review recommended actions before escalation. A site manager overseeing multiple lines can compare patterns across facilities to understand recurring bottlenecks. An operational excellence lead can use SAMI to examine long-term trends, compare lines or plants, and prioritize improvement opportunities with clearer evidence. These role-based examples align with the scenarios outlined in the provided material and illustrate how AI-supported collaboration functions during daily operations.
Equally important is the structured nature of the workspace. Tier Boards align operators, supervisors, and leadership around shared priorities and real-time metrics, while SAMI ensures that analysis is linked to the specific events and records under discussion. That integration supports faster handovers, clearer accountability, and more consistent application of lessons learned across shifts. Because the system captures both structured and unstructured data, SAMI can draw on the institutional knowledge stored in shift notes and logs—often the missing context when teams try to reconstruct what happened and why.
The platform’s use by global pharmaceutical manufacturers underscores its suitability for GMP-driven environments, where traceability, data integrity, and audit readiness are essential. By embedding AI within role-specific workflows and tying insights back to source records, the launch shows how collaborative AI can support operational performance and compliance without adding unnecessary complexity to the workday.

“We are honored to be recognized in not just one, but two categories in the Pharmaceutical Technology Excellence Awards. This recognition highlights the impact of our Shiftconnector® Tier Collaboration Dashboards in transforming operational data visibility, and the innovation behind SAMI in advancing intelligent manufacturing insights. It’s a testament to the dedication of the entire eschbach team and fuels our drive to continue evolving as an intelligent operational platform for the pharmaceutical industry.”
– Andreas Eschbach, CEO of eschbach
Company Profile
eschbach is a software development company specializing in plant process management solutions for the process industry.
eschbach’s flagship product, Shiftconnector®, is a no-code, SaaS platform designed to integrate seamlessly with existing plant operation systems like SAP and Aveva PI. Shiftconnector® centralizes operational data, streamlines team communication, and supports real-time decision-making, thereby enhancing efficiency and safety in 24/7 plant operations. The platform is utilized by leading manufacturing companies across 21 countries and is available in eight languages.
eschbach is committed to continuous innovation, as evidenced by the development of AI-driven features like Smart Search, which leverages artificial intelligence to provide production teams with process-relevant knowledge in a user-friendly format. The company holds certifications such as ISO 9001 for quality management and ISO 27001 for information security management, underscoring its dedication to quality and security in its solutions.
Founded in 2005, the company is headquartered in Bad Säckingen, Germany, with a North American office in Boston, Massachusetts.
Donna Jenkins
Head, Marketing and Communications – North America
E-mail: donna.jenkins@eschbach.com
Direct: +1 614-571-0758
Links
Website: www.eschbach.com