October 07, 2025
2 min read
Key takeaways:
- After 2 years of tildrakizumab, participants with psoriasis reported psychological well-being scores higher than the general population.
- POSITIVE is the first dermatology study to use the WHO-5 Well-Being Index.
Sustained two-year treatment with tildrakizumab improved the psychological well-being of people with moderate to severe plaque psoriasis, as well as their partners, according to a study.
The POSITIVE study is a prospective, observational, real-world phase 4 trial that evaluated the overall well-being of people who are being treated with tildrakizumab (Ilumetri, Almirall/Ilumya, Sun Pharmaceutical Industries), an anti-interleukin-23 p19 biologic indicated for moderate to severe plaque psoriasis. The initial two-year results from this study were presented at the 2025 European Academy of Dermatology and Venerology Congress.

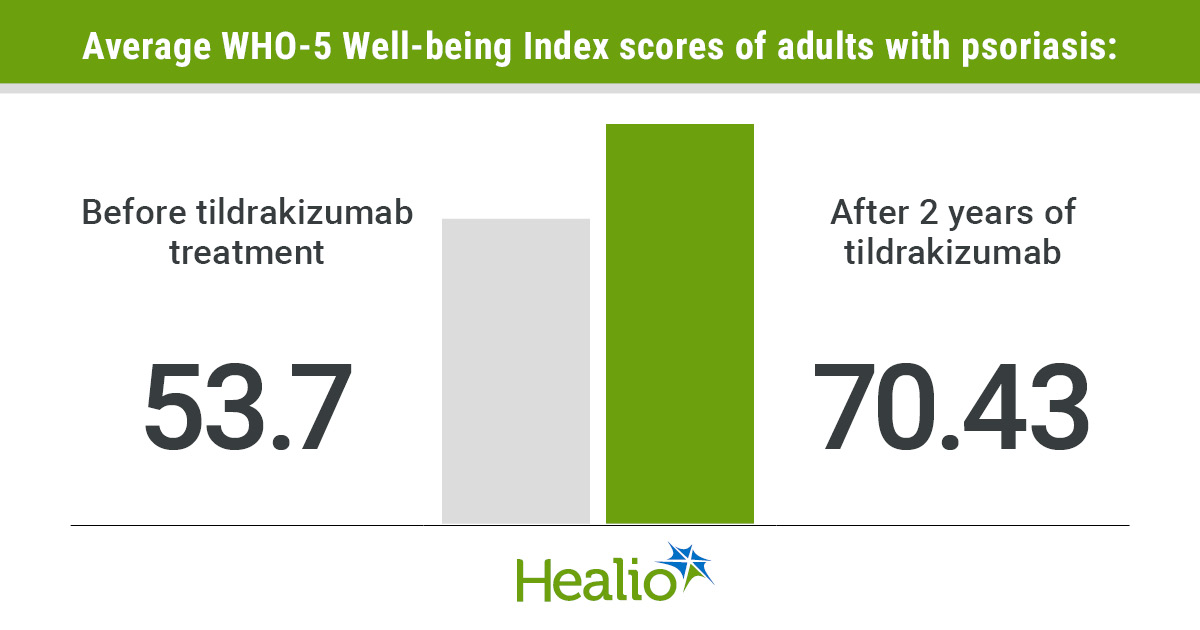
Data derived from Mrowietz U, et al. Abstract #LBA-305. Presented at: European Academy of Dermatology and Venereology Congress; Sept. 17-20, 2025; Paris.
“When it comes to psoriasis, we now have a range of drugs that have a very good clinical efficacy, with the majority of patients achieving skin clearance,” Ulrich Mrowietz, MD, professor of dermatology and founder of the Psoriasis Center at the University Medical Center Schleswig-Holstein as well as lead investigator of POSITIVE, told Healio. “However, there has been fairly little emphasis on the patients themselves.”

Ulrich Mrowietz
For the study, researchers used the WHO-5 Well-Being Index, a five-item questionnaire that assesses the psychological well-being of patients undergoing treatment. Scores range from zero to 100, with higher scores reflecting better well-being. This study marks the first time the WHO-5 has been used as a primary endpoint in a dermatology study, as Healio previously reported.
The study included 785 adults (mean age, 46.9 years; 35.3% women) across nine European countries who were receiving tildrakizumab for moderate to severe plaque psoriasis. In contrast to the general population’s psychological well-being score of 64.9, the participants with psoriasis in the study averaged 53.7 (P < .0001) — a score comparable to that of breast cancer patients (52.2). However, after receiving 16 weeks of treatment, the average psychological well-being score was 63.2. By 2 years, the average well-being score of adults receiving tildrakizumab for psoriasis reached 70.43, exceeding the general population’s average psychological well-being score, according to study data.
The participants’ PASI improved from 12.9 at baseline to 1.3 at year 2, with 79% of patients maintaining a PASI that was less than 2, indicating psoriasis control, by the end of the study. Their revised DLQI also decreased from 12 at baseline to 2.1 at year 2.
“The study not only assessed the patient’s psychological well-being, but we also looked into how the partners of people who have psoriasis are affected by the disease and how treatment benefited them,” Mrowietz said. “We found that there was significant improvement in a partner’s burden as well.”
Measured by the FamilyPsO scale, a 15-item questionnaire that measures the burden experienced by partners and family members of people with psoriasis, the partners’ average baseline score of 1.1 decreased to 0.6 after 2 years of treatment with tildrakizumab (P < .001).
This finding shows how a treatment’s impact for chronic dermatologic conditions extends beyond the skin, Mrowietz said.
“It is about the regaining of overall well-being,” Mrowietz told Healio. “It’s [about] restoring the life of the patients and the patients’ partners. This will impact patient care in the future.”
Notably, 29.5% of patients reported “psycholag,” defined as a delayed psychological recovery despite rapid skin clearance. Mrowietz said this could be due to patients and their partners remaining skeptical of disease recurrence, highlighting the complexities of emotional recovery and the need for dermatologists to assess mental health outcomes.
For more information:
Ulrich Mrowietz, MD, can be reached at umrowietz@dermatology.uni-kiel.de.