Shots:
- CuraTeQ Biologics, a subsidiary of Aurobindo Pharma, reported positive P-III results for its denosumab biosimilar targeting postmenopausal osteoporosis, as an alternative to Prolia
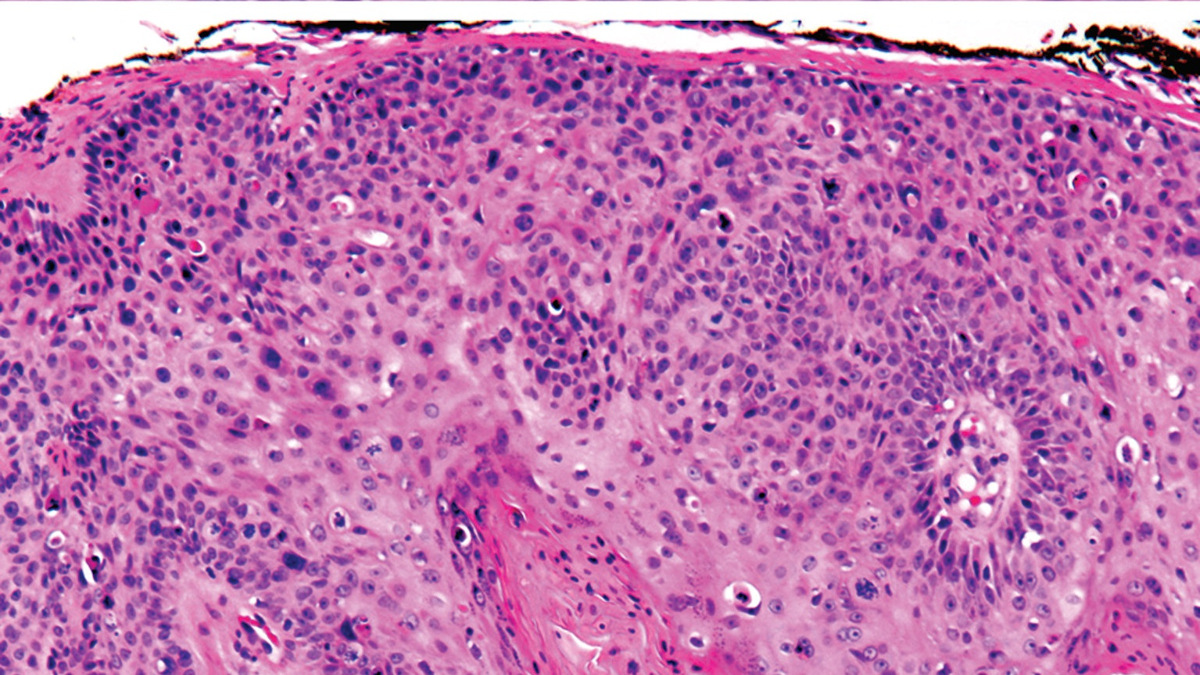
- The study involved 446 postmenopausal women across 40 sites in EU met all EPs, showing no significant differences between the biosimilar and Prolia
- The trial confirmed the biosimilar’s efficacy in improving bone density and reducing fracture risk. It met the 1EP (LS-BMD % change at Wk. 52 within -1.45 to +1.45) and co-primary EP (sCTX AUEC from Wk. 0 to Wk. 26 within 0.80–1.25), meeting FDA and EMA criteria
Ref: CuraTeQ | Image: CuraTeQ | Press Release
Related News:- The EC Approves CuraTeQ Biologics’ Dazublys (Biosimilars, Herceptin)
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com