Image Credit: Adobe Stock Images/Марина Терехова
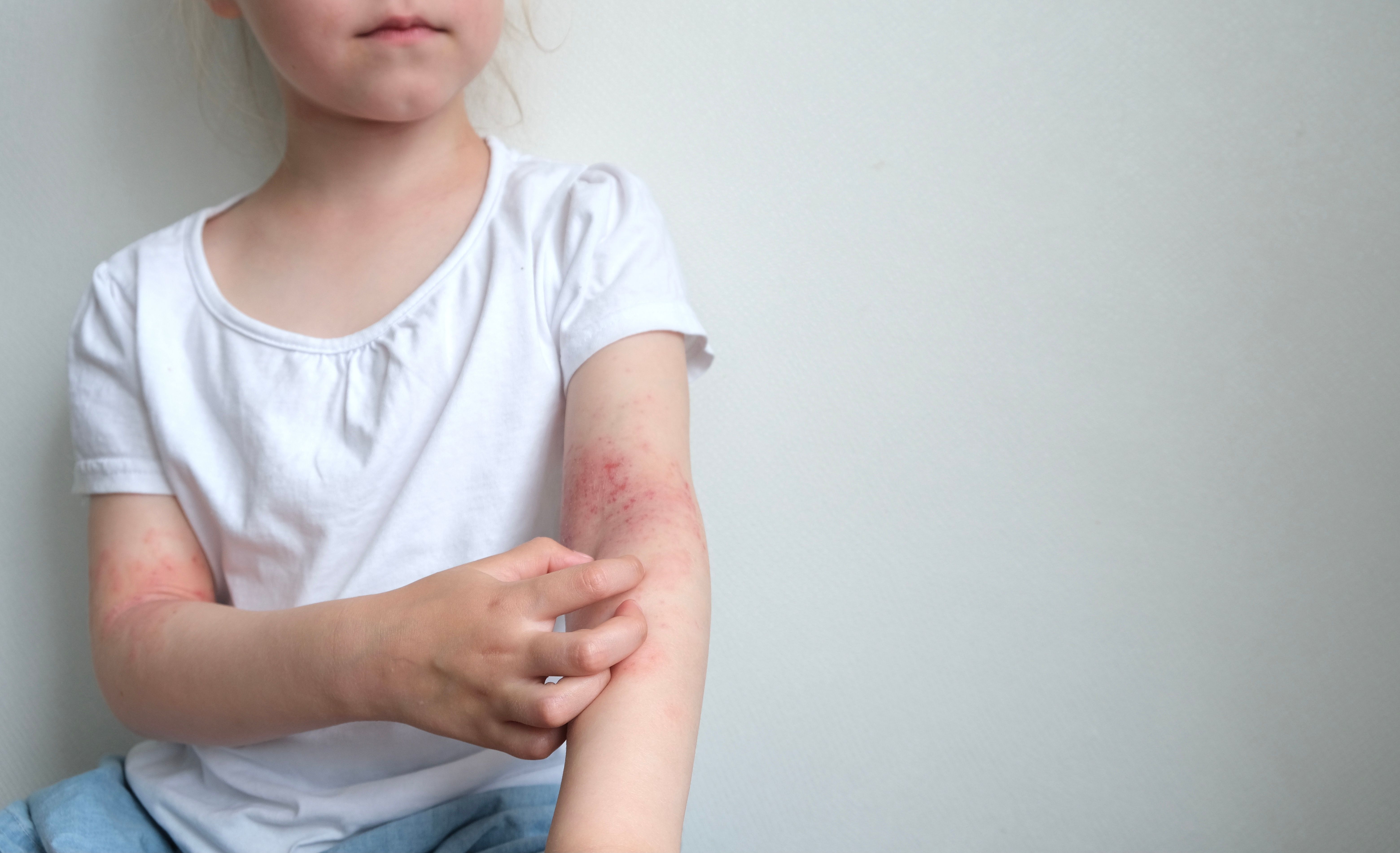
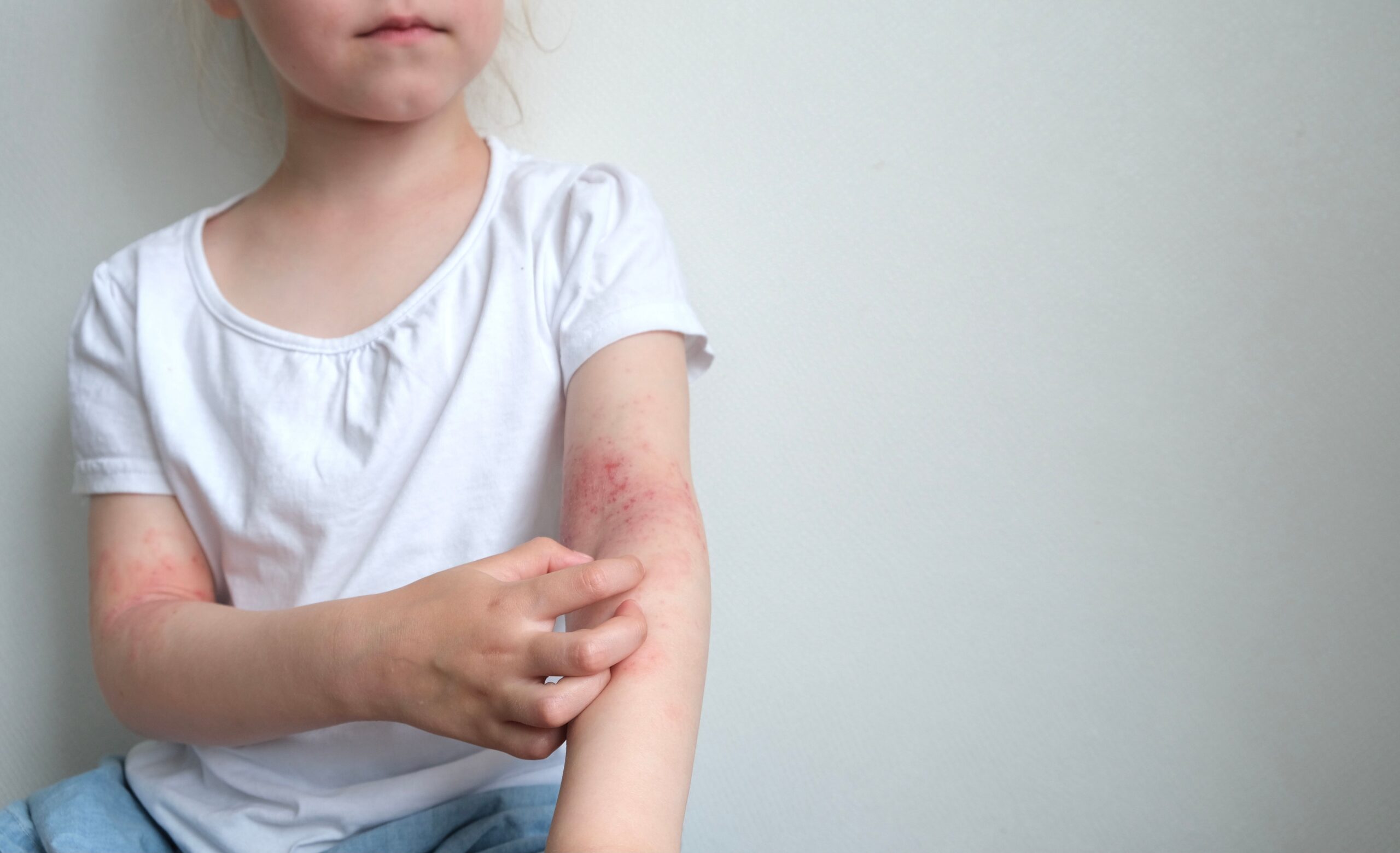
The FDA has approved an expanded indication for Incyte’s Opzelura (ruxolitinib) cream to include children as young as two years of age with mild to moderate atopic dermatitis (AD).1
The regulatory action, based on positive data from Phase III TRuE-AD3 trial (NCT04921969),2 marks the first steroid-free topical treatment option indicated for this age group.
“With this approval, we now have a new, non-steroidal topical option that expands how we care for kids with this chronic disease. This is a meaningful step forward and marks a significant advancement in our ability to better support our pediatric patients.”
“Navigating a complex condition like atopic dermatitis can be very challenging for children, who currently have limited treatment options to meet their specific needs,” Peter Lio, MD, clinical assistant professor of Dermatology and Pediatrics at Northwestern University Feinberg School of Medicine, said in a press release. “With this approval, we now have a new, non-steroidal topical option that expands how we care for kids with this chronic disease. This is a meaningful step forward and marks a significant advancement in our ability to better support our pediatric patients.”1
- Opzelura, which inhibits both the JAK1 and JAK2 pathways, was initially granted FDA approval in September 2021 for the treatment of patients aged 12 years or older with mild to moderate AD who did not respond to prior therapies.3
- AD, which is the most common form of eczema, affects approximately 16.5 million adults and 9.6 million children in the United States, according to the National Eczema Association.
- An estimated 80% of patients with AD develop the condition before reaching six years of age; while one in four adults develop their first symptoms later in life.4
“While every child’s journey with AD is unique, for many, the skin redness and irritation can profoundly impact their well being,” Korey Capozza, founder of Global Parents for Eczema Research, said in the release. “When you’re managing a condition that can affect daily life, access to safe, effective, and age-appropriate options is critical. With limited, safe treatment options currently available, especially for younger children, the addition of new therapies that control symptoms is so important to meet the needs and goals for children with AD and their families.”1
Phase III TRuE-AD3 trial results show strong efficacy and safety
- The randomized, double-blind, vehicle-controlled TRuE-AD3 trial compared the safety and efficacy of Opzelura vs. non-medicated cream in children between two and 12 years of age with AD.
- Investigators enrolled patients who were diagnosed with AD for a minimum of three months and who are candidates for topical therapy.
- Inclusion criteria included an Investigator’s Global Assessment (IGA) score of 2 to 3 and presence of AD on 3% to 20% of body surface area not including the scalp.
- A total of 330 patients were randomly assigned in a 2:2:1 ratio to receive Opzelura 0.75% (n = 134) administered twice daily (BID); Opzelura 1.5% (n = 131) BID; or non-medicated cream BID (n = 65).
- Those who were able to complete an efficacy assessment after eight weeks were allowed to participate in a long-term safety treatment extension period of 44-weeks receiving the same dosage strength of Opzelura at 0.75% or 1.5% BID.
- Patients who initially received non-medicated cream were again randomly assigned, this time in a blinded 1:1 ratio, to either of the Opzelura cohorts.
- The trial’s primary endpoint was proportion of patients who achieved IGA Treatment Success (IGA-TS), which was defined clear or almost clear skin with at least a two-point improvement from baseline at week eight.
- Secondary endpoints included proportion of patients achieving at least a 75% improvement in the Eczema Area and Severity Index (EASI-75), proportion of patients between six and 12 years of age with at least a four-point improvement in itch numerical rating scale (NRS) based on NRS4 at week eight and time to achieve NRS4; and frequency, duration, and severity of adverse events (AEs) associated with Opzelura use.
Clinical improvements with Opzelura observed as early as week two
- Results show that at week eight, 36.6% and 56.5% of patients administered Opzelura 0.75% and 1.5% achieved IGA-TS, respectively, compared to 10.8% in the non-medicated cream cohort (P = .0001/P < .0001).5
- Patients also reported improvements in itch and quality of life.
- A total of 51.5% of patients in the 0.75% Opzelura cohort and 67.2% in the 1.5% Opzelura cohort achieved EASI-75 compared to 15.4% in the in the non-medicated cream cohort (both P < .0001) at week eight.
- A total of 34.3% of patients in the 0.75% Opzelura cohort and 43.5% in the 1.5% Opzelura cohort achieved EASI-90 compared to 10.8% in the non-medicated cream cohort (P = .0004 and P < .0001, respectively) at week eight.
- Investigators reported clinical improvements in IGA-TS and EASI responses across both Opzelura dose strengths compared to non-medicated cream as early as week two, with continued improvement through week eight.
Safety profile consistent with prior trials in older patients
- In terms of safety, AEs were mild or moderate in nature and did not require treatment interruption or discontinuation.
- The most commonly reported AEs were upper respiratory tract infection and nasopharyngitis.
Expanded approval addresses critical unmet need in pediatric dermatology
“With this approval, we are now able to offer younger children with atopic dermatitis and their families a much-needed, steroid-free topical treatment option with the potential to significantly improve the burdensome symptoms they experience every day,” said Bill Meury, CEO, Incyte, in the release. “At Incyte, we are committed to delivering innovative solutions that address every stage of a patient’s journey; this approval is another step toward addressing the real-world challenges faced by patients suffering from chronic skin conditions, including people living with atopic dermatitis.”1
References
1. Incyte Announces Additional FDA Approval of Opzelura® (Ruxolitinib) Cream in Children Ages 2-11 with Atopic Dermatitis. News release. Incyte. September 18, 2025. Accessed September 19, 2025. https://investor.incyte.com/news-releases/news-release-details/incyte-announces-additional-fda-approval-opzelurar-ruxolitinib
2. A Study to Assess the Efficacy and Safety of Ruxolitinib Cream in Children With Atopic Dermatitis (TRuE-AD3). ClinicalTrials.gov. Updated March 28, 2025. Accessed September 19, 2025. https://www.clinicaltrials.gov/study/NCT04921969
3. Opzelura. Package insert. AbbVie; 2022. Accessed September 19, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
4. Eczema Stats. NEA. Accessed September 19, 2025. https://nationaleczema.org/eczema-facts/
5. Efficacy and safety of ruxolitinib cream in children aged 2 to 11 years with atopic dermatitis: Results from TRuE-AD3, a phase 3, randomized double-blind study. Eichenfield, Lawrence F. et al. Journal of the American Academy of Dermatology, Volume 93, Issue 3, 689 – 698.