September 08, 2025
2 min read
Key takeaways:
- Azelastine lowered the risk for SARS-CoV-2 infection vs. placebo.
- The spray may have potential as an adjunct to reduce COVID-19 risk but vaccination “remains the cornerstone” of prevention, a researcher said.
Azelastine nasal spray was associated with a reduced risk for SARS-CoV-2 infection, according to the results of a phase 2 randomized controlled trial published in JAMA Internal Medicine.
In the trial, healthy German adults were assigned 1:1 to either azelastine 0.1% (n = 227) or placebo (n = 223) three times daily for 56 days.

Azelastine lowered the risk for SARS-CoV-2 infection vs. placebo. Image: Adobe Stock
Robert Bals, MD, PhD, from the department of internal medicine and pneumology at Saarland University Medical Center in Germany, and colleagues noted that SARS-CoV-2 rapid antigen testing was conducted twice weekly, with positive results confirmed by polymerase chain reaction (PCR).
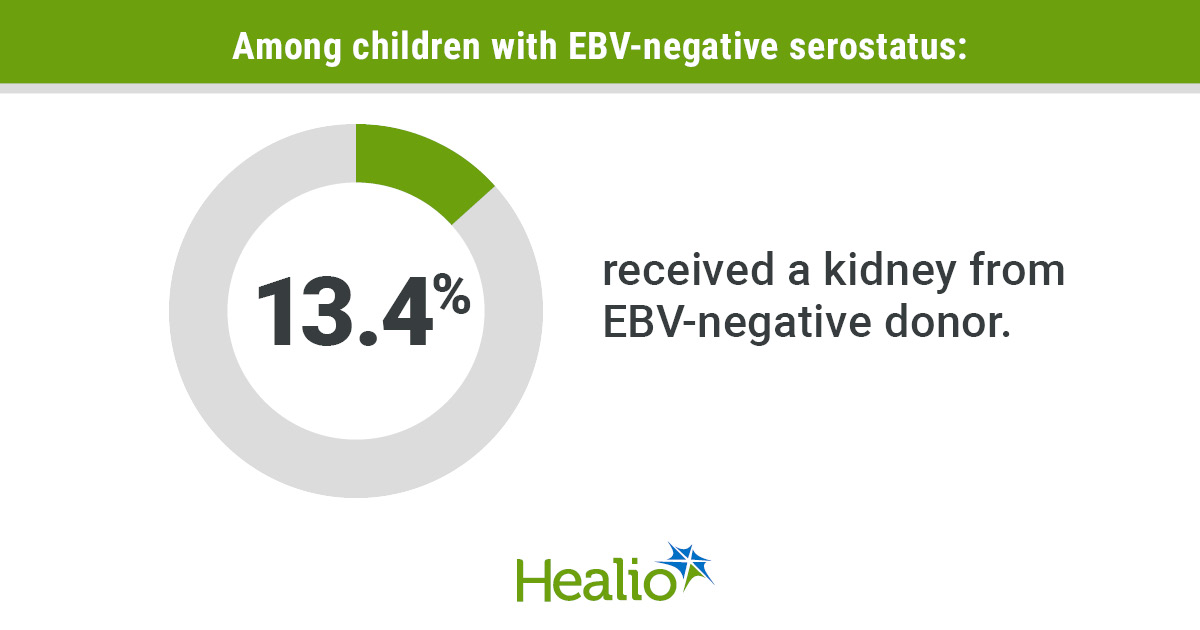
The researchers reported that the incidence of PCR-confirmed SARS-CoV-2 infection was substantially lower in the azelastine group compared with the placebo group (OR = 0.31; 95% CI, 0.11-0.87).
“The established safety profile, over-the-counter availability and ease of use of azelastine nasal spray support its potential as a practical, scalable on demand approach to preexposure prophylaxis, particularly in high-risk settings such as large gatherings or travel,” the researchers wrote.
Healio spoke with Bals to learn more about the possible underlying mechanisms behind the findings, what role azelastine could play in the prevention of COVID-19 and more.
Healio: Can you elaborate on the potential mechanisms underlying the antiviral activity of azelastine? How strong is the evidence?
Bals: Azelastine has demonstrated in vitro activity against SARS-CoV-2 and other respiratory viruses. The proposed mechanisms are not entirely clear but include interference with viral entry (via angiotensin-converting enzyme 2), inhibition of the viral main protease, modulation of host sigma-1 receptor signaling and suppression of intercellular adhesion molecule 1, which rhinoviruses exploit. The mechanistic evidence is strong at the preclinical level, but the precise contribution of each pathway in humans remains to be clarified.
Healio: Were the study participants previously vaccinated against COVID-19?
Bals: Yes, almost all participants had received at least one COVID-19 vaccination, with a median of three doses.
Healio: Will future studies examine the effects of azelastine in vaccinated vs. unvaccinated participants?
Bals: Future trials should stratify participants by vaccination status, prior infection and risk profile to better define efficacy across different immunity backgrounds.
Healio: If azelastine continues to show promise, what role would it have in COVID-19 prevention? When would patients use it?
Bals: If confirmed, azelastine could serve as an easily accessible, over-the-counter adjunct to reduce infection risk — particularly during periods of high viral circulation, in crowded settings, or for people at elevated risk. The exact conditions need to be studied in clinical trials.
Healio: Do you think azelastine will supplement vaccination? Or serve as an alternative?
Bals: Azelastine would be considered a supplementary measure. Vaccination remains the cornerstone of COVID-19 prevention. Nasal prophylaxis may add an extra layer of protection, but it cannot substitute for systemic immunity.
Healio: What are the next steps of this research?
Bals: We are discussing multicenter, larger-scale clinical trials in more diverse populations to confirm efficacy, clarify benefits and assess its potential against a broader spectrum of respiratory viruses.
Reference:
For more information:
Robert Bals, MD, PhD, can be reached at primarycare@healio.com.