August 21, 2025
2 min read
Key takeaways:
- JAK inhibition carried higher malignancy risk vs. biologic DMARDs.
- Malignancy risk was seen in older patients and those with high disease activity.
JAK inhibitors for rheumatoid arthritis demonstrate higher risks for cancer vs. biologic disease-modifying antirheumatic drugs, particularly among older patients and those with higher disease activity, according to data.
“The Oral Rheumatoid Arthritis Trial (ORAL) Surveillance study included a risk-enriched population of RA patients with a minimum age of 50 years and 1 cardiovascular (CV) risk factor,” Martin Schaefer, MD, of the German Rheumatology Research Center, in Berlin, and colleagues wrote in the Annals of the Rheumatic Diseases. “It detected an overall malignancy risk (excluding nonmelanoma skin cancer) of 1.47 (95% CI: 1.00, 2.18) for 5 mg tofacitinib twice daily compared to tumor necrosis factor inhibitors (TNFis), prompting the [FDA] and the [EMA] to issue warnings regarding tofacitinib use.

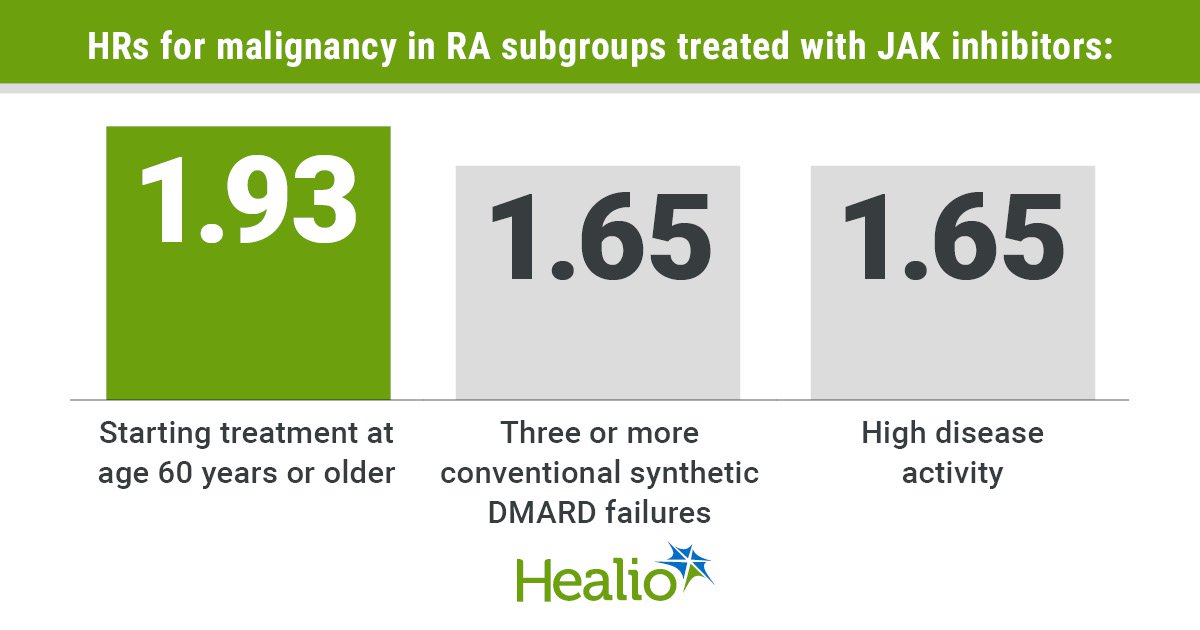
Data were derived from Schaefer M, et al. Ann Rheum Dis. 2025;doi:10.1016/j.ard.2025.05.014.
“Several studies using register or claims data of RA patients have attempted to replicate the ORAL Surveillance (OS) findings in real-world settings,” they added. “… It remains largely unclear what the differences in the results of the individual studies can be attributed to.”
To examine the effects of JAK inhibitors vs. biologic DMARDs on the risk for malignancies, excluding nonmelanoma skin cancer, in patients with RA, Schaefer and colleagues assessed data from the German RABBIT registry. According to the researchers, RABBIT was created to observe and follow RA patients being treated with biologic and targeted DMARDs. Included participants initiated DMARD therapy between January 2017 and December 2020 and were followed through June 2024.
According to the researchers, there were 88 malignancies reported among 2,285 patients receiving JAK inhibitors, and 135 malignancies reported in 4,295 patients receiving biologic DMARDs. Baricitinib (Olumiant, Eli Lilly) and tofacitinib (Xeljanz, Pfizer) were the most commonly used JAK inhibitors, while TNF inhibitors were most common among those receiving biologic DMARDs.
The incidence ratio for malignancy risk in the JAK inhibitor group was 11.6 per 1,000 patient-years (95% CI, 9.3-14.3), compared with 8.9 per 1,000 patient years (95% CI, 7.4-10.5) for biologic DMARDS. The increase in malignancy risk associated with JAK inhibitors compared with other biologic DMARDs was only observed in patients treated for longer than 16 months, according to the findings.
Malignancy risk was higher in three particular subgroups of RA patients, including those who initiated treatment at age 60 years or older (HR = 1.93; 95% CI, 1.43-2.6), those with three or more prior conventional synthetic DMARD treatments (HR = 1.65; 95% CI, 1.08-2.52), and those with high disease activity (HR = 1.65; 95% CI, 1.02-2.7).
“Treatment with JAK [inhibitors] (predominantly baricitinib and tofacitinib) was associated with an increased HR of malignancies compared to treatment with [biologic] DMARDs in the overall study cohort, consistent with results from the OS trial,” Schaefer and colleagues wrote. “The increase in the HR could only be detected in treatment episodes with follow-up lasting longer than 16 months.
“… A more pronounced risk increase could be detected for some patient groups, including older patients starting treatment aged 60 years, patients with 3 [conventional synthetic] DMARD failures, as well as patients with high disease activity,” they added. “This can help physicians to assess the individual risk of their patients and thus strengthen precision medicine, as well as to decide which patients should be more closely monitored.”
For more information:
Healio Rheumatology can be reached at rheumatology@healio.com.