August 19, 2025
4 min read
Key takeaways:
- AAP and CDC vaccine recommendations have been mostly harmonized for 30 years.
- AAP guidance now deviates from the CDC’s on COVID-19 and influenza vaccines.
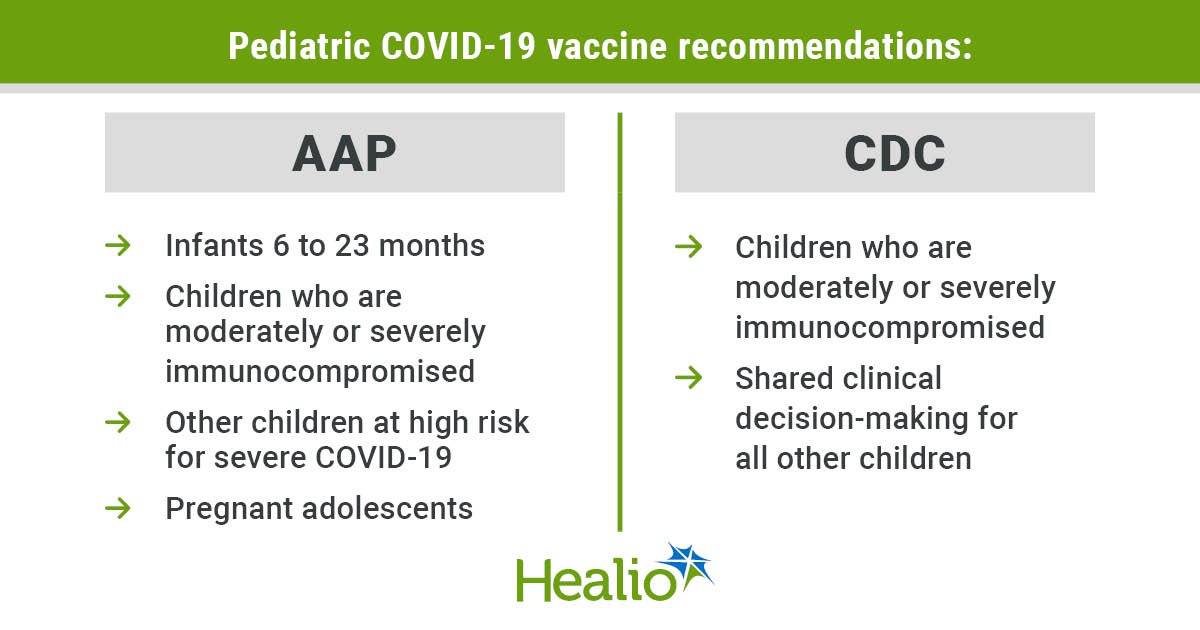
The American Academy of Pediatrics on Tuesday recommended that all infants get vaccinated against COVID-19, deviating from the CDC on that and several other recommendations in its updated immunization schedule for children and adolescents.
It was a rare split from the CDC, which no longer universally recommends COVID-19 vaccination for kids. The AAP also broke with federal recommendations on thimerosal-containing influenza vaccines and COVID-19 vaccination in pregnant adolescents.

Derived from AAP and CDC.
“Since its founding in 1930, the American Academy of Pediatrics has been a leading voice in vaccine recommendations, creating evidence-based guidance to support pediatricians in caring for children and families,” the AAP said in a press release, declaring that the new schedule “continues in this tradition.”
“It differs from recent recommendations of the Advisory Committee on Immunization Practices (ACIP) of the CDC, which was overhauled this year and replaced with individuals who have a history of spreading vaccine misinformation,” the AAP said.
COVID-19 vaccines
Earlier this year, the CDC tweaked its guidelines for pediatric COVID-19 vaccines from a universal recommendation to a shared clinical decision-making model for children aged 6 months through 17 years who are not moderately or severely immunocompromised.
The change came after HHS Secretary Robert F. Kennedy Jr. had said the CDC would stop recommending COVID-19 shots for healthy children and healthy pregnant women altogether.
In contrast, the AAP recommends that all infants aged 6 through 23 months receive a COVID-19 vaccine, as well as children aged 2 to 18 years in certain risk groups — including those who are at high risk for severe disease themselves or who live in a household with high-risk contacts, and those who have never been vaccinated against COVID-19.
Additionally, the AAP recommends COVID-19 vaccination for pregnant adolescents, who are not included in CDC recommendations.
Both the AAP and CDC recommend COVID-19 vaccination for anyone aged 6 months or older who is moderately or severely immunocompromised.
“It is extremely important that all families who want their kids to have the benefit of COVID-19 vaccination be able to access the vaccine,” Adam J. Ratner, MD, MPH, a member of the AAP Committee on Infectious Diseases, told Healio.
Healio Pediatrics Peer Perspective Board Member Paul A. Offit, MD, director of the Vaccine Education Center and attending physician in the division of infectious diseases at The Children’s Hospital of Philadelphia, said physicians should follow the AAP recommendations because children — especially those aged younger than 2 years — are at a high risk for severe COVID-19.
“The AAP schedule is based on reported data from last year that there have been thousands of hospitalizations … that 20% of those kids were admitted to the intensive care unit, and that 152 children died,” Offit told Healio.
He said Kennedy “stood up in May of this year and unilaterally declared that HHS was not going to recommend this vaccine for healthy young children based on no data.”
According to KFF, most insurance payers are required to cover the cost of vaccines recommended by the CDC, even under the shared decision-making model for kids whose physician determines they should receive it based on individual risk factors.
“It is absolutely critical that insurers continue to provide coverage for recommended vaccines,” Ratner said. “The AAP is working with other groups to make sure that all children have access to vaccination.”
Flu and RSV
The AAP’s vaccine recommendations for respiratory diseases include one other notable difference from the CDC.
After the ACIP said in June that the U.S. should not administer influenza vaccines containing thimerosal, a safe preservative, the AAP added a recommendation that “influenza vaccination … not be delayed to obtain a specific product, including a thimerosal-free product.”
“The CDC recommendation to avoid multi-dose formulations that contain thimerosal as a preservative is not based on scientifically valid studies,” Ratner said.
Otherwise, both sets of recommendations say everyone aged 6 months or older should get an influenza vaccine.
The recommendations are also aligned for immunization against respiratory syncytial virus, which is recommended for infants younger than age 8 months and toddlers aged 8 through 19 months at high risk for severe RSV who are entering their second RSV season.
The monoclonal antibody clesrovimab was approved by the FDA earlier this month, joining a growing list of available options to protect adults and infants from RSV, which include multiple vaccines, a maternal vaccine and nirsevimab, another mAb.
Usually in harmony
The AAP and CDC immunization schedules have mostly been harmonized since 1995. One exception was when they split on recommending the influenza nasal spray vaccine.
Neither recommended the nasal spray during the 2016-2017 or 2017-2018 influenza seasons, citing low effectiveness in previous seasons. However, the ACIP voted to make it available during the 2018-2019 season after new data showed it performed better in protecting people from influenza. The AAP, on the other hand, recommended the injectable vaccine over the nasal spray that season, noting that the nasal spray should only be used as a “last resort.”
References:
- AAP. Recommended child and adolescent immunization schedule for ages 18 years or younger. https://publications.aap.org/redbook/resources/15585. Updated Aug. 19, 2025. Accessed Aug. 19, 2025.
- AAP influenza immunization recommendations revised for 2018-19 season. https://publications.aap.org/aapnews/news/6697/AAP-influenza-immunization-recommendations-revised. Published June 7, 2018. Accessed Aug. 19, 2025.
- American Academy of Pediatrics releases influenza vaccine recommendations for 2025-26 season. https://www.aap.org/en/news-room/news-releases/aap/2025/american-academy-of-pediatrics-releases-influenza-vaccine-recommendations-for-2025-26-season/. Published July 28, 2025. Accessed Aug. 19, 2025.
- CDC. ACIP Recommendations. https://www.cdc.gov/acip/vaccine-recommendations/index.html. Updated Aug. 8, 2025. Accessed Aug. 19, 2025.
- KFF. ACIP, CDC and insurance coverage of vaccines in the United States. https://www.kff.org/other/issue-brief/acip-cdc-and-insurance-coverage-of-vaccines-in-the-united-states/. Published June 13, 2025. Accessed Aug. 19, 2025.
- The American Academy of Pediatrics releases its own evidence-based immunization schedule. https://www.aap.org/en/news-room/news-releases/aap/2025/the-american-academy-of-pediatrics-releases-its-own-evidence-based-immunization-schedule/. Published Aug. 19, 2025. Accessed Aug. 19, 2025.
For more information:
Adam J. Ratner, MD, MPH, and Paul A. Offit, MD, can be reached at pediatrics@healio.com.