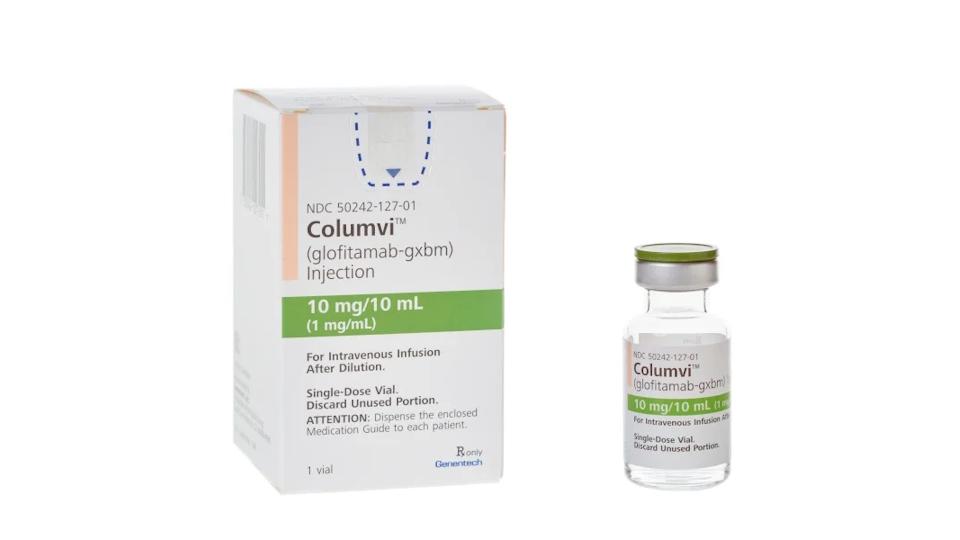
Thousands of people in England and Wales with a form of non-Hodgkin lymphoma (NHL) could soon have access to a new treatment on the NHS – Roche’s Columvi – if their cancer has returned or didn’t respond to initial treatment.
Reimbursement authority NICE published draft guidance today giving a green light to the use of Columvi (glofitamab) in combination with gemcitabine and oxaliplatin chemotherapy as a second-line option for adults with diffuse large B-cell lymphoma (DLBCL) who are ineligible for a stem cell transplant.
Columvi, a CD20xCD3 bispecific antibody, became the first drug in the class to be approved in Europe for this indication earlier this year, allowing it to be used earlier in treatment compared to rivals like AbbVie and Genmab’s rival CD20xCD3 bispecific Epkinly (epcoritamab).
People whose DLBCL had relapsed or stopped responding to treatment often have limited options, and bispecific antibodies like Columvi and Epkinly offer an off-the-shelf and easier-to-administer alternative to cell-based CAR-T therapies for DLBCL, which have complex manufacturing and dosing requirements.
Around 5,500 people are diagnosed with DLBCL in the UK each year, and while many respond well to initial therapy, for those whose cancer returns, the chances of survival are poor.
Tracey Loftis, deputy director of policy and influencing at patient advocacy group Blood Cancer UK, said the draft guidance represents “a real step forward” for people living with DLBCL.
“Blood cancer is the UK’s fifth most common cancer and the UK’s third biggest cancer killer. That’s why new treatments like glofitamab are so important, as is the research and importantly clinical trials that are required to get them to people affected by blood cancer,” she added.
“Even as new treatments become available, getting a timely blood cancer diagnosis is critical. Blood cancer symptoms like persistent tiredness, night sweats, weight loss or swollen lymph nodes should never be ignored.”
Roche has also submitted the drug to the Scottish Medicines Consortium (SMC) but is still waiting on a date for the appraisal.
“This decision is a significant milestone, providing a much-needed option for progressed patients in England and Wales,” said Pia Ballschmieter, UK haematology lead at Roche Products Ltd.
“We are committed to making this treatment available across the rest of the UK as quickly as possible.”
Roche recently reported global sales of Columvi rose more than 80% to around $250 million in the first nine months of 2025, with the increase driven by second-line use.